Search Thermo Fisher Scientific
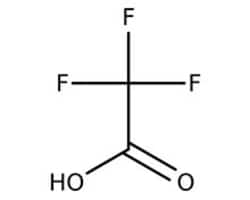
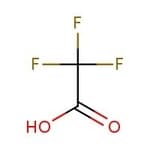
Trifluoroacetic Acid
Trifluoracetic acid is an organofluorine compound that is a structural analogue of acetic acid with all three of the acetyl group’s hydrogen atoms replaced by fluorine atoms. It is stronger than acetic acid, having an acid ionization constant of pKA 0.23. Trifluoracetic acid is available in various quantities and reagent grades. It is widely used in organic synthesis, mass spectrometry, and NMR spectroscopy.
Products (13)
Learn More (0)
Documents & Support
(746)13 Products
Filter
The combination of properties like solubility in most of the solvents, volatility, catalytic property, and strong acidity with non-oxidizing nature makes it a widely used reagent in organic synthesis. Trifluoroacetic acid is an important building block in the synthesis of pharmaceuticals,...
C2HF3O2, CAS Number-76-05-1, acetic acid, trifluoro, cf3cooh, kyselina trifluoroctova, perfluoroacetic acid, trifluoroacetic acid, trifluoroaceticacid, trifluoro acetic acid, trifluoro-acetic acid, trifluoroacetic acid, trifluoroethanoic acid, 100mL, 72 deg.C, CHEBI:45892, Colorless, 114.02g/mol
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
The combination of properties like solubility in most of the solvents, volatility, catalytic property, and strong acidity with non-oxidizing nature makes it a widely used reagent in organic synthesis. Trifluoroacetic acid is an important building block in the synthesis of pharmaceuticals,...
The combination of properties like solubility in most of the solvents, volatility, catalytic property and strong acidity with non-oxidizing nature makes it a widely used reagent in an organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
Trifluoroacetic Acid, >-99.5%, C2HF3O2, CAS Number-76-05-1, trifluoroethanoic acid, trifluoroacetic acid, cf3cooh, acetic acid, trifluoro, trifluoro-acetic acid, perfluoroacetic acid, kyselina trifluoroctova, trifluoro acetic acid, trifluoroacetic acid, trifluoroaceticacid, 50mL
The combination of properties like solubility in most of the solvents, volatility, catalytic property and strong acidity with non-oxidizing nature makes it a widely used reagent in an organic synthesis.