Search Thermo Fisher Scientific
Thermo Scientific Chemicals
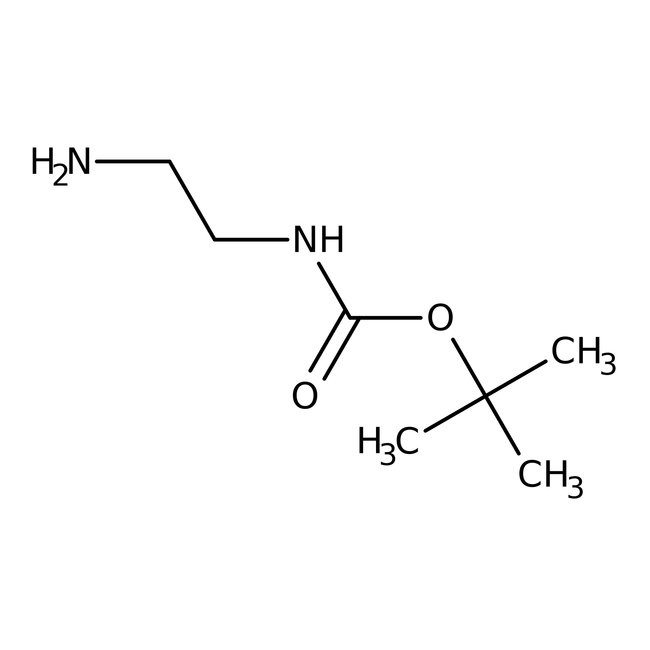
N-Boc-ethylenediamine, 98%, may cont up to 5% tert-butanol
CAS: 57260-73-8 | C7H16N2O2 | 160.22 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL19947.06 | 5 g |
Catalog number ALFL19947.06
Price (MYR)
750.00
EA
Quantity:
5 g
Price (MYR)
750.00
EA
Specifications
Chemical Name or MaterialN-Boc-ethylenediamine
CAS57260-73-8
Boiling Point72°C (0.1mmHg)
Health Hazard 1H314-H335-H336
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
View more
N-Boc-ethylenediamine is used in the synthesis of thyronamine derivatives. It is also used in the preparation of (2-isothiocyanato-ethyl)-carbamic acid tert-butyl ester by reacting with carbon disulfide. Further, it is used in both oligonucleotide and peptide synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Boc-ethylenediamine is used in the synthesis of thyronamine derivatives. It is also used in the preparation of (2-isothiocyanato-ethyl)-carbamic acid tert-butyl ester by reacting with carbon disulfide. Further, it is used in both oligonucleotide and peptide synthesis.
Solubility
Miscible with methanol and chloroform. Slightly miscible with water.
Notes
Air sensitive. Store in a cool place. Incompatible with strong oxidizing agents and acids.
N-Boc-ethylenediamine is used in the synthesis of thyronamine derivatives. It is also used in the preparation of (2-isothiocyanato-ethyl)-carbamic acid tert-butyl ester by reacting with carbon disulfide. Further, it is used in both oligonucleotide and peptide synthesis.
Solubility
Miscible with methanol and chloroform. Slightly miscible with water.
Notes
Air sensitive. Store in a cool place. Incompatible with strong oxidizing agents and acids.
RUO – Research Use Only
General References:
- Zhang, Q.; Jin, B.; Peng, R.; Lei, S.; Chu, S. Symmetrical 1,3-dicarbonyl biscatecholamide ligands as sequestering agents for uranyl decorporation. Polyhedron 2015, 87, 417-423.
- Nasr, F. H.; Khoee, S. Design, characterization and in vitro evaluation of novel shell crosslinked poly(butylene adipate)-co-N-succinyl chitosan nanogels containing loteprednol etabonate: A new system for therapeutic effect enhancement via controlled drug delivery. Eur. J. Med. Chem. 2015, 102, 132-142.