Search Thermo Fisher Scientific
Thermo Scientific Chemicals
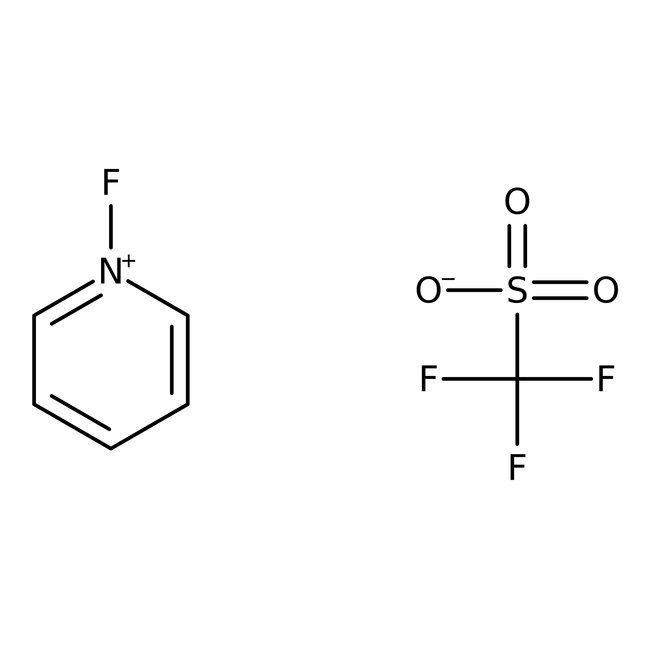
N-Fluoropyridinium trifluoromethanesulfonate, 95%
CAS: 107263-95-6 | C6H5F4NO3S | 247.164 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL14324.03 | 1 g |
Catalog number ALFL14324.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or MaterialN-Fluoropyridinium trifluoromethanesulfonate
CAS107263-95-6
Health Hazard 1H314-H335
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P271-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
N-Fluoropyridinium trifluoromethanesulfonate is used as a reagent for electrophilic fluorination and for a review of electrophilic N-F fluorinating agents. It is also used as a reactant for Pd-catalyzed arylation reactions and as a fluorination agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Fluoropyridinium trifluoromethanesulfonate is used as a reagent for electrophilic fluorination and for a review of electrophilic N-F fluorinating agents. It is also used as a reactant for Pd-catalyzed arylation reactions and as a fluorination agent.
Solubility
Reacts with water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. It is sensitive to moisture. Incompatible with oxidizing agents. Store under dry inert gas.
N-Fluoropyridinium trifluoromethanesulfonate is used as a reagent for electrophilic fluorination and for a review of electrophilic N-F fluorinating agents. It is also used as a reactant for Pd-catalyzed arylation reactions and as a fluorination agent.
Solubility
Reacts with water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. It is sensitive to moisture. Incompatible with oxidizing agents. Store under dry inert gas.
RUO – Research Use Only
General References:
- Xisheng Wang.; Dasheng Leow.; Jin-Quan Yu. Pd(II)-catalyzed para-selective C-H arylation of monosubstituted arenes. Journal of the American Chemical Society. 2011, 133 (35), 13864-13867
- Teruo Umemoto.; Kosuke Kawada.; Kyoichi Tomita. N-fluoropyridinium triflate and its derivatives: Useful fluorinating agents. Tetrahedron Letters. 1986, 27 (37), 4465-4468
- Reagent for electrophilic fluorination:
- Alkyl or silyl enol ethers give ɑ-fluoro ketones: Tetrahedron Lett., 27, 3271, 4465 (1986); Org. Synth. Coll., 8, 286 (1993). For regioselective mono-ɑ-fluorination of steroids bearing several silyl enol ether groups, see: J. Am. Chem. Soc., 112, 8563 (1990).
- For a review of electrophilic N-F fluorinating agents, see: Chem. Rev., 96, 1737 (1996).