Search Thermo Fisher Scientific
Thermo Scientific Chemicals
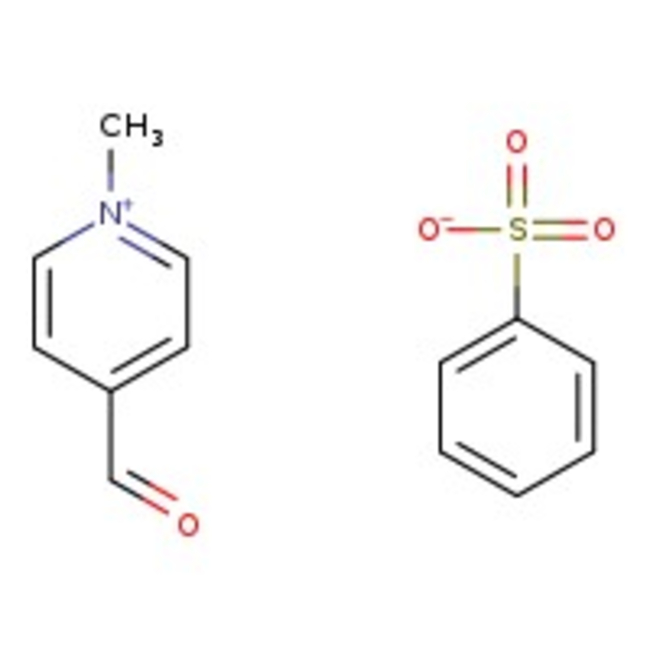
N-Methylpyridinium-4-carboxaldehyde benzenesulfonate hydrate, 97%
CAS: 82228-89-5 | C13H13NO4S | 279.31 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL12072.06 | 5 g |
Catalog number ALFL12072.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or MaterialN-Methylpyridinium-4-carboxaldehyde benzenesulfonate hydrate
CAS82228-89-5
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
N-Methylpyridinium-4-carboxaldehyde benzenesulfonate hydrate is a useful reagent for the chemical transformation of primary amines to aldehydes and ketones under extremely mild conditions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Methylpyridinium-4-carboxaldehyde benzenesulfonate hydrate is a useful reagent for the chemical transformation of primary amines to aldehydes and ketones under extremely mild conditions.
Solubility
Sparingly soluble in water (0.27 g/L at 25°C).
Notes
Keep container tightly closed in a dry and well-ventilated place. It is hygroscopic in nature. Store under inert gas. Incompatible with oxidizing agents.
N-Methylpyridinium-4-carboxaldehyde benzenesulfonate hydrate is a useful reagent for the chemical transformation of primary amines to aldehydes and ketones under extremely mild conditions.
Solubility
Sparingly soluble in water (0.27 g/L at 25°C).
Notes
Keep container tightly closed in a dry and well-ventilated place. It is hygroscopic in nature. Store under inert gas. Incompatible with oxidizing agents.
RUO – Research Use Only
Reagent for conversion of primary amines to the corresponding aldehydes and ketones under extremely mild conditions. The reaction mimics the action of pyridoxal in biological systems and is compatible with various functional groups: J. Am. Chem. Soc., 104, 4446 (1982).