Search Thermo Fisher Scientific
Thermo Scientific Chemicals
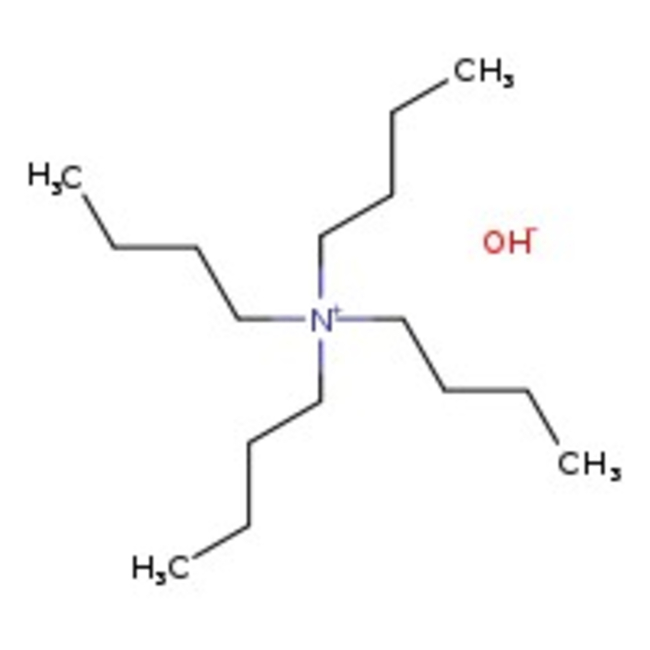
Tetra-n-butylammonium hydroxide, 1.0M in methanol
CAS: 2052-49-5 | C16H37NO | 259.48 g/mol
Catalog number ALFL08164.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or MaterialTetra-n-butylammonium hydroxide
CAS2052-49-5
Health Hazard 1H225-H301+H311+H331-H314-H335-H370
Health Hazard 2GHS H Statement
H225-H301-H311-H331-H370-H314-H318
Highly flammable liquid and vapor.
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes damage to organs.
Causes severe skin burns and eye damage.
Causes serious eye damage.
H225-H301-H311-H331-H370-H314-H318
Highly flammable liquid and vapor.
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes damage to organs.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Tetra-n-butylammonium hydroxide, 1.0M in methanol is used as a strong base in organic chemistry. It is utilized under phase-transfer conditions to effect alkylation and deprotonation.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetra-n-butylammonium hydroxide, 1.0M in methanol is used as a strong base in organic chemistry. It is utilized under phase-transfer conditions to effect alkylation and deprotonation.
Solubility
Miscible with organic solvents.
Notes
Air sensitive and hygroscopic. Incompatible with strong acids and corrodes metal.
Tetra-n-butylammonium hydroxide, 1.0M in methanol is used as a strong base in organic chemistry. It is utilized under phase-transfer conditions to effect alkylation and deprotonation.
Solubility
Miscible with organic solvents.
Notes
Air sensitive and hygroscopic. Incompatible with strong acids and corrodes metal.
RUO – Research Use Only
General References:
- Li, S. H.; Li, Z. J.; Nakagawa, T.; Ryan, J. W.; Matsuo, Y.; Gao, X. Approach to High Open-Circuit Voltage in Organic Solar Cells Utilizing a Structural Change of the Oxazolino-C70 Derivative. Chem. Eur. J. 2015, 21 (5), 1894-1899.
- Ou, Z.; Meng, D.; Yuan, M.; Huang, W.; Fang, Y.; Kadish, K. M. Deprotonation Reactions and Electrochemistry of Substituted Open-Chain Pentapyrroles and Sapphyrins in Basic Nonaqueous Media. J. Phys. Chem. B 2013, 117 (43), 13646-13657.