Search Thermo Fisher Scientific
Thermo Scientific Chemicals
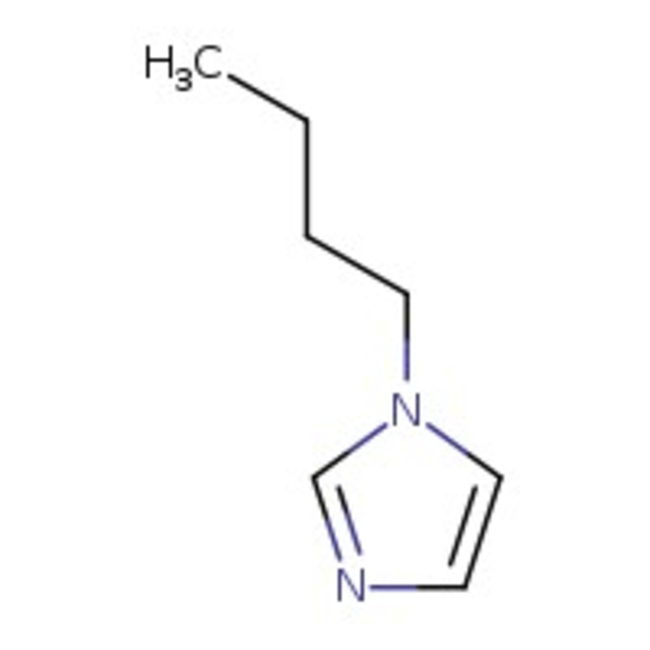
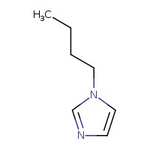
1-(n-Butyl)imidazole, 99%
CAS: 4316-42-1 | C7H12N2 | 124.19 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL07793.14 | 25 g |
Catalog number ALFL07793.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material1-(n-Butyl)imidazole
CAS4316-42-1
Health Hazard 1H315-H318-H330-H335
Health Hazard 2GHS H Statement
H330-H318-H315-H335
Fatal if inhaled.
Causes serious eye damage.
Causes skin irritation.
May cause respiratory irritation.
H330-H318-H315-H335
Fatal if inhaled.
Causes serious eye damage.
Causes skin irritation.
May cause respiratory irritation.
Health Hazard 3P260-P264b-P271-P280-P284-P302+P352-P304+P340-P305+P351+P338-P310-P312-P332+P313-P362-P501c
View more
1-(n-Butyl)imidazole is used in the synthesis of a heterocyclic mesomeric betaine and 1-(1-butyl-3-imidazolio)propane-3-sulfonate (BIm3S). It acts as an N-coordinated ligand. It is used in the chiral separation of amino acids and anionic pharmaceuticals by capillary electrophoresis. It is used for preparation of substituted phenyl-containing imidazolium ionic liquid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-(n-Butyl)imidazole is used in the synthesis of a heterocyclic mesomeric betaine and 1-(1-butyl-3-imidazolio)propane-3-sulfonate (BIm3S). It acts as an N-coordinated ligand. It is used in the chiral separation of amino acids and anionic pharmaceuticals by capillary electrophoresis. It is used for preparation of substituted phenyl-containing imidazolium ionic liquid.
Solubility
Soluble in Chloroform, Methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible materials are oxidizing agents.
1-(n-Butyl)imidazole is used in the synthesis of a heterocyclic mesomeric betaine and 1-(1-butyl-3-imidazolio)propane-3-sulfonate (BIm3S). It acts as an N-coordinated ligand. It is used in the chiral separation of amino acids and anionic pharmaceuticals by capillary electrophoresis. It is used for preparation of substituted phenyl-containing imidazolium ionic liquid.
Solubility
Soluble in Chloroform, Methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible materials are oxidizing agents.
RUO – Research Use Only
General References:
- Nina Gonsior; Fabian Mohr; Helmut Ritter. Synthesis of mesomeric betaine compounds with imidazolium-enolate structure.Beilstein Journal of Organic Chemistry.2012, 8 390-397.
- G M Smith; G G Duncan. A study of intravascular platelet aggregation by continuous platelet counting.Thrombosis Research.1981, 23 (3), 275-288.
- Preferred ligand in a Cu(I)-catalyzed procedure for cyanation of heteroaryl bromides with potassium hexacyanoferrate(II), as an alternative to conventional routes with highly toxic metal cyanides: Synlett, 555 (2007).