Search Thermo Fisher Scientific
Thermo Scientific Chemicals
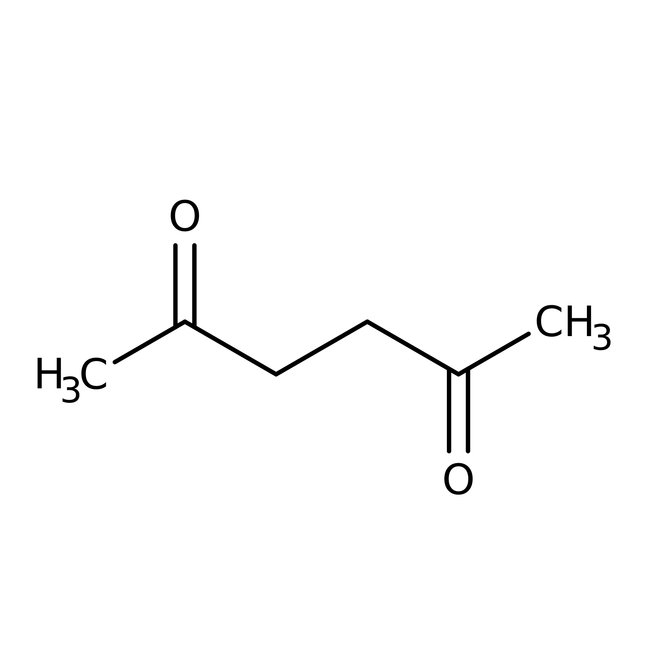
2,5-Hexanedione, 97%
CAS: 110-13-4 | C6H10O2 | 114.144 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB25686.14 | 25 g |
Catalog number ALFB25686.14
Price (MYR)
365.00
EA
Quantity:
25 g
Price (MYR)
365.00
EA
Specifications
Chemical Name or Material2,5-Hexanedione
CAS110-13-4
Health Hazard 1H227-H315-H319-H373
Health Hazard 2GHS H Statement
H361-H373-H302-H332-H315-H319-H335-H227
Suspected of damaging fertility or the unborn child.
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H361-H373-H302-H332-H315-H319-H335-H227
Suspected of damaging fertility or the unborn child.
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P260-P264b-P280-P302+P352-P305+P351+P338-P314-P332+P313-P362-P370+P378q-P501c
View more
2,5-Hexanedione is used as a reagent in the preparation of trans-2,5-dimethylpyrrolidine. It is also used in the synthesis of 2,5-dimethylpyrroles. Further, it plays an important role as a reagent used for the protection of amino groups in amino sugars and nucleosides. In addition to this, it is used in the preparation of five-membered heterocycles like indane-type and benzannulated systems. It is also employed as a precursor in Diels-alder cycloaddition reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,5-Hexanedione is used as a reagent in the preparation of trans-2,5-dimethylpyrrolidine. It is also used in the synthesis of 2,5-dimethylpyrroles. Further, it plays an important role as a reagent used for the protection of amino groups in amino sugars and nucleosides. In addition to this, it is used in the preparation of five-membered heterocycles like indane-type and benzannulated systems. It is also employed as a precursor in Diels-alder cycloaddition reactions.
Solubility
Miscible with alcohol, water and diethyl ether.
Notes
Incompatible with strong oxidizing agents, strong bases, metals and strong reducing agents.
2,5-Hexanedione is used as a reagent in the preparation of trans-2,5-dimethylpyrrolidine. It is also used in the synthesis of 2,5-dimethylpyrroles. Further, it plays an important role as a reagent used for the protection of amino groups in amino sugars and nucleosides. In addition to this, it is used in the preparation of five-membered heterocycles like indane-type and benzannulated systems. It is also employed as a precursor in Diels-alder cycloaddition reactions.
Solubility
Miscible with alcohol, water and diethyl ether.
Notes
Incompatible with strong oxidizing agents, strong bases, metals and strong reducing agents.
RUO – Research Use Only
General References:
- Primary amines can be protected by conversion to 2,5-dimethylpyrroles, from which they can be released by reaction with hydroxylamine hydrochloride: J. Chem. Soc., Perkin 1, 2801 (1984). The method has been found useful for protecting amino sugars in oligosaccharide synthesis: J. Org. Chem., 63, 4570 (1998), and also in the protection of an arylamine during Cu(I) promoted methoxylation of a ring bromo or iodo substituent; Synthesis, 1599 (1998).
- Sacia, E. R.; Deaner, M. H.; Louie, Y. L.; Bell, A. T. Synthesis of biomass-derived methylcyclopentane as a gasoline additivevia aldol condensation/hydrodeoxygenation of 2,5-hexanedione. Green Chem. 2015, 17 (4), 2393-2397.
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Conversion of cellulose to 2,5-hexanedione using tungstated zirconia in hydrogen atmosphere. Appl. Catal., A 2015, 504, 664-671.