Search Thermo Fisher Scientific
Thermo Scientific Chemicals
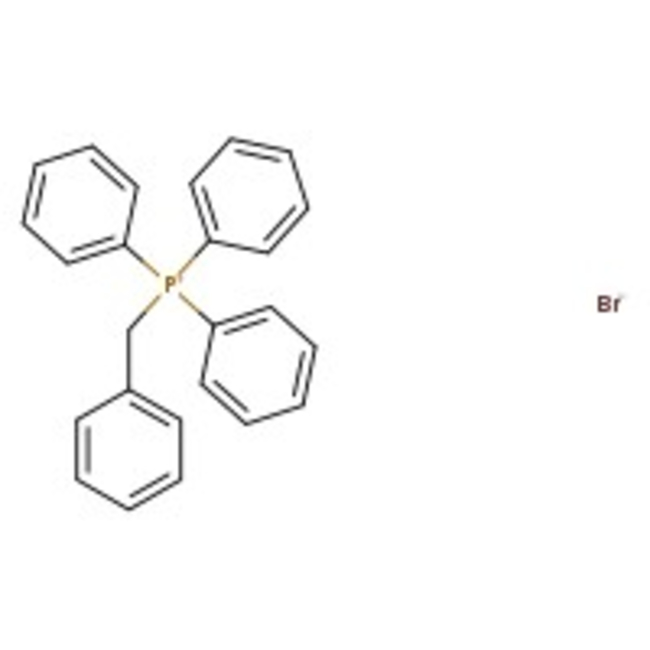
Benzyltriphenylphosphonium bromide, 98%
CAS: 1449-46-3 | C25H22BrP | 433.33 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB24567.18 | 50 g |
Catalog number ALFB24567.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or MaterialBenzyltriphenylphosphonium bromide
CAS1449-46-3
Health Hazard 1H302+H312+H332
Health Hazard 2GHS H Statement
H300-H315-H319-H335
Fatal if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H300-H315-H319-H335
Fatal if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P312-P330-P363-P501c
View more
Benzyltriphenylphosphonium bromide is used as a reactant for stereoselective azidolysis of vinyl epoxides, enantioselective aziridination and Friedel-Crafts cyclization for asymmetric synthesis of dihydrexidine, biomimetic iron(III) mediated oxidative dimerization for synthesis of benzoquinone parvistemin A, enantioselective synthesis of syn-diarylheptanoids from D-glucose, preparation of β-amyloid plaque ligands and decarboxylative cyclopropanation. It react with 4-methyl-oxetan-2-one to produce 4-hydroxy-1-phenyl-1-(triphenyl-l5-phosphanylidene)-pentan-2-one.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Benzyltriphenylphosphonium bromide is used as a reactant for stereoselective azidolysis of vinyl epoxides, enantioselective aziridination and Friedel-Crafts cyclization for asymmetric synthesis of dihydrexidine, biomimetic iron(III) mediated oxidative dimerization for synthesis of benzoquinone parvistemin A, enantioselective synthesis of syn-diarylheptanoids from D-glucose, preparation of β-amyloid plaque ligands and decarboxylative cyclopropanation. It react with 4-methyl-oxetan-2-one to produce 4-hydroxy-1-phenyl-1-(triphenyl-l5-phosphanylidene)-pentan-2-one.
Solubility
Soluble in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
Benzyltriphenylphosphonium bromide is used as a reactant for stereoselective azidolysis of vinyl epoxides, enantioselective aziridination and Friedel-Crafts cyclization for asymmetric synthesis of dihydrexidine, biomimetic iron(III) mediated oxidative dimerization for synthesis of benzoquinone parvistemin A, enantioselective synthesis of syn-diarylheptanoids from D-glucose, preparation of β-amyloid plaque ligands and decarboxylative cyclopropanation. It react with 4-methyl-oxetan-2-one to produce 4-hydroxy-1-phenyl-1-(triphenyl-l5-phosphanylidene)-pentan-2-one.
Solubility
Soluble in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Saumen Hajra, Sukanta Bar. Catalytic enantioselective synthesis of A-86929, a dopamine D1 agonist. Chemical Communications. 2011, 47 (13), 3981-3982.
- Marcus J Smith, Christopher C Nawrat, Christopher J Moody. Synthesis of parvistemin A via biomimetic oxidative dimerization. Organic Letters. 2011, 13 (13), 3396-3398.
- Wittig reaction of benzylic phosphonium salts with aromatic aldehydes using solid KOH + 18-crown-6 leads to (Z)-stilbenes with good stereoselectivity: Tetrahedron Lett., 37, 4225 (1996). See Appendix 1.