Search Thermo Fisher Scientific
Thermo Scientific Chemicals
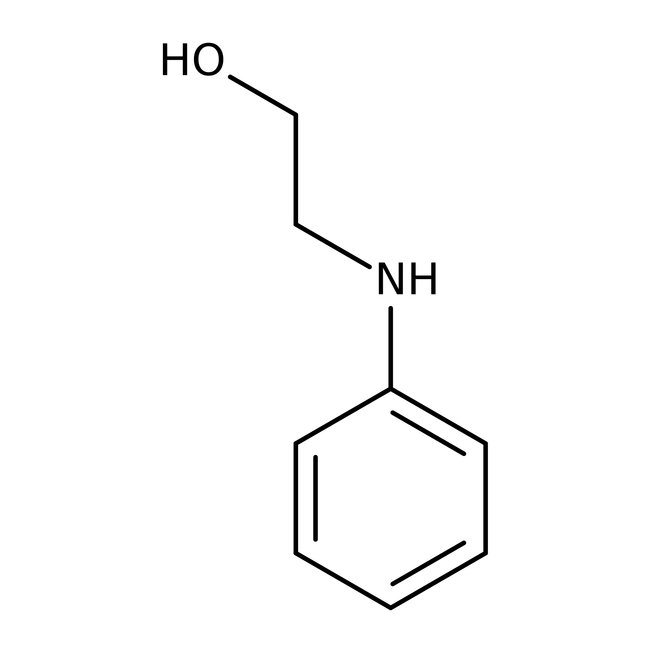
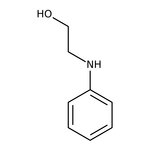
N-Phenylethanolamine, 98%
CAS: 122-98-5 | C8H11NO | 137.182 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB22131.36 | 500 g |
Catalog number ALFB22131.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialN-Phenylethanolamine
CAS122-98-5
Health Hazard 1H311-H319
Health Hazard 2GHS H Statement
H310-H351-H373-H319-H317
Fatal in contact with skin.
Suspected of causing cancer.
May cause damage to organs through prolonged or repeated exposure.
Causes serious eye irritation.
May cause an allergic skin reaction.
H310-H351-H373-H319-H317
Fatal in contact with skin.
Suspected of causing cancer.
May cause damage to organs through prolonged or repeated exposure.
Causes serious eye irritation.
May cause an allergic skin reaction.
Health Hazard 3P264b-P280-P302+P352-P305+P351+P338-P312-P361-P363-P501c
View more
N-Phenylethanolamine is used as an intermediate for dyes and other organic compounds. Further, it is used as a substrate for human olfactory uridine 5'-diphospho-glucuronosyltransferase.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
N-Phenylethanolamine is used as an intermediate for dyes and other organic compounds. Further, it is used as a substrate for human olfactory uridine 5′-diphospho-glucuronosyltransferase.
Solubility
Slightly miscible with water.
Notes
Incompatible with acids, acid anhydrides, acid chlorides and chloroformates.
N-Phenylethanolamine is used as an intermediate for dyes and other organic compounds. Further, it is used as a substrate for human olfactory uridine 5′-diphospho-glucuronosyltransferase.
Solubility
Slightly miscible with water.
Notes
Incompatible with acids, acid anhydrides, acid chlorides and chloroformates.
RUO – Research Use Only
General References:
- Wei, X.; Wan, S.; Jiang, X.; Wang, Z.; Gao, S. Peanut-Shell-like Porous Carbon from Nitrogen-Containing Poly-N-phenylethanolamine for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7 (40), 22238-22245.
- Li, C.; Liu, Y.; Zhang, Y.; Zhang, X. An approach to the anticoagulant agent rivaroxaban via an isocyanate-oxirane cycloaddition promoted by MgI2.etherate. J. Chem. Res. 2011, 35 (7), 400-401.
- Stanicki, D.; Pottier, M.; Gantois, N.; Pinçon, C.; Forge, D.; Mahieu, I.; Boutry, S.; Eynde, J. J. V.; Martinez, A.; Dei-Cas, E.; Aliouat, E. M. Diamidines versus Monoamidines as Anti-Pneumocystis Agents: An in Vivo Study. Pharmaceuticals 2013, 6 (7), 837-850.