Search Thermo Fisher Scientific
Thermo Scientific Chemicals
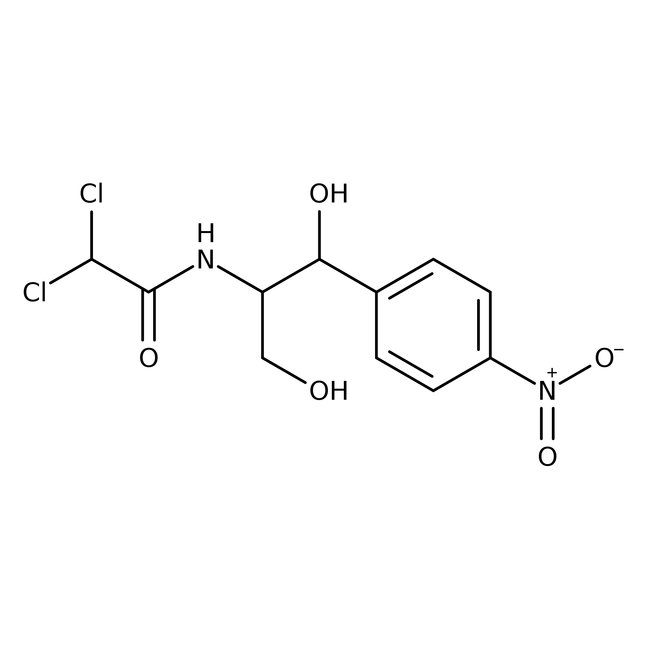
(R,R)-Chloramphenicol, 98%
(R,R)-Chloramphenicol, CAS # 56-75-7, also known as chloromycetin, is a compound with broad-spectrum antibiotic and bacteriostatic activities. | CAS: 56-75-7 | C11H12Cl2N2O5 | 323.126 g/mol
Catalog number ALFB20841.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material(R,R)-Chloramphenicol
Type(R,R)-Chloramphenicol
Physical FormCrystalline Powder
CAS56-75-7
Health Hazard 1H350
View more
Inhibitor of translation on the 50S subunit at the peptidyltransferase step. (R,R)-Chloramphenicol is widely utilized as an antibiotic, which is used for the treatment of number of bacterial infections and also act as a bacteriostatic. It is often used for bacterial selection in molecular biology applications. It also finds an application in ophthalmic and veterinary purposes. It also protects the mitochondrial and chloroplast protein synthesis and ribosomal formation of (p)ppGpp, de-pressing rRNA transcription.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
- (R,R)-chloramphenicol is a compound with antibiotic and bacteriostatic activity and is derived from Streptomyces venequelae
- It that can block the translation on the 50S subunit at the peptidyltransferase step. The binding affects the peptidyl transferase activity, preventing the transfer of amino acids to the growing peptide chains and inhibiting peptide bond formation. The resulting inhibition of the bacterial protein synthesis impedes bacterial cell proliferation
Application
- (R,R)-chloramphenicol has been used for several molecular biology approaches, mainly for bacterial selection. It can be used to select transformed cells containing chloramphenicol resistance genes
- This compound can also promote mitochondrial and chloroplast protein synthesis and the ribosomal formation of (p)ppGpp, depressing the rRNA transcription process
- In vitro results demonstrate that (R,R)-chloramphenicol shows activity against several vancomycin-resistant E. faecium strains
RUO – Research Use Only
General References:
- Fedeniuk, R. W.; Mizuno, M.; Neiser, C.; O’Byrne, C. Development of LC-MS/MS methodology for the detection/determination and confirmation of chloramphenicol, chloramphenicol 3-O- β-d-glucuronide, florfenicol, florfenicol amine and thiamphenicol residues in bovine, equine and porcine liver. J. Chromatogr. B 2015, 991, 68-78.
- Mitchell, S. M.; Ullman, J. L.; Teel, A. L.; Watts, R. J. Hydrolysis of amphenicol and macrolide antibiotics: Chloramphenicol, florfenicol, spiramycin, and tylosin. Chemosphere 2015, 134, 504-511.