Search Thermo Fisher Scientific
Thermo Scientific Chemicals
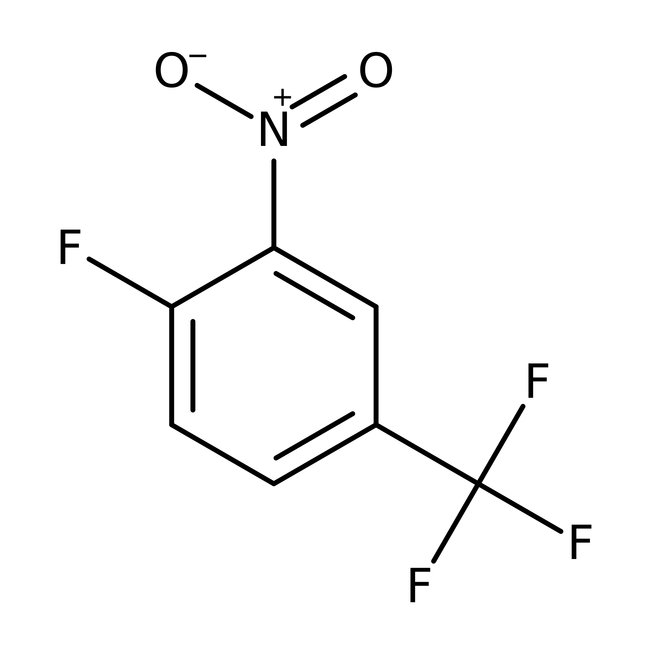
4-Fluoro-3-nitrobenzotrifluoride, 97%
CAS: 367-86-2 | C7H3F4NO2 | 209.1 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA19676.14 | 25 g |
Catalog number ALFA19676.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material4-Fluoro-3-nitrobenzotrifluoride
CAS367-86-2
Health Hazard 1H226-H315-H319-H335
Health Hazard 2GHS H Statement
H311-H227-H302-H332-H315-H319
Toxic in contact with skin.
Combustible liquid.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
H311-H227-H302-H332-H315-H319
Toxic in contact with skin.
Combustible liquid.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P312-P332+P313-P363-P370+P378q-P501c
View more
4-Fluoro-3-nitrobenzotrifluoride is used in the pre-column derivatization technique for HPLC with UV/VIS spectrophotometric detection of polyamines. It may be used as derivatization reagent for the HPLC determination of polyamines. It was also used in the synthesis of 2-nitro-4-trifluoromethylphenylhydrazine, derivatization reagent for 1,2-diol compounds and oxo-steroids.
- [3-(2-nitro-4-trifluoromethylphenyl)aminophenyl]dihydroxyborane, derivatization reagent for 1,2-diol compounds and oxo-steroids3
- 1-(3-chlorophenyl)-5-trifluoromethyl-3-hydrobenzimidazol-2-one
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Fluoro-3-nitrobenzotrifluoride is used in the pre-column derivatization technique for HPLC with UV/VIS spectrophotometric detection of polyamines. It may be used as derivatization reagent for the HPLC determination of polyamines. It was also used in the synthesis of 2-nitro-4-trifluoromethylphenylhydrazine, derivatization reagent for 1,2-diol compounds and oxo-steroids. • [3-(2-nitro-4-trifluoromethylphenyl)aminophenyl]dihydroxyborane, derivatization reagent for 1,2-diol compounds and oxo-steroids3 • 1-(3-chlorophenyl)-5-trifluoromethyl-3-hydrobenzimidazol-2-one
Solubility
Insoluble in water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
4-Fluoro-3-nitrobenzotrifluoride is used in the pre-column derivatization technique for HPLC with UV/VIS spectrophotometric detection of polyamines. It may be used as derivatization reagent for the HPLC determination of polyamines. It was also used in the synthesis of 2-nitro-4-trifluoromethylphenylhydrazine, derivatization reagent for 1,2-diol compounds and oxo-steroids. • [3-(2-nitro-4-trifluoromethylphenyl)aminophenyl]dihydroxyborane, derivatization reagent for 1,2-diol compounds and oxo-steroids3 • 1-(3-chlorophenyl)-5-trifluoromethyl-3-hydrobenzimidazol-2-one
Solubility
Insoluble in water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- Jorgen Vessman.; Signhild Stromberg. Determination of tranexamic acid in biological material by electron capture gas chromatography after direct derivatization in an aqueous medium. Anal. Chem. 1977, 49, (3), 369-373
- J. T. Gerig.; J. D. Reinheimer. Modification of human serum albumin with trifluoromethyl-substituted aryl halides and sulfonates J. Am. Chem. Soc. 1975, 97, (1), 168-173
- The nitro-group can be selectively displaced with KF in sulfolane, in the presence of phthaloyl chloride as a nitrite trap: J. Org. Chem., 56, 6406 (1991).