Search Thermo Fisher Scientific
Thermo Scientific Chemicals
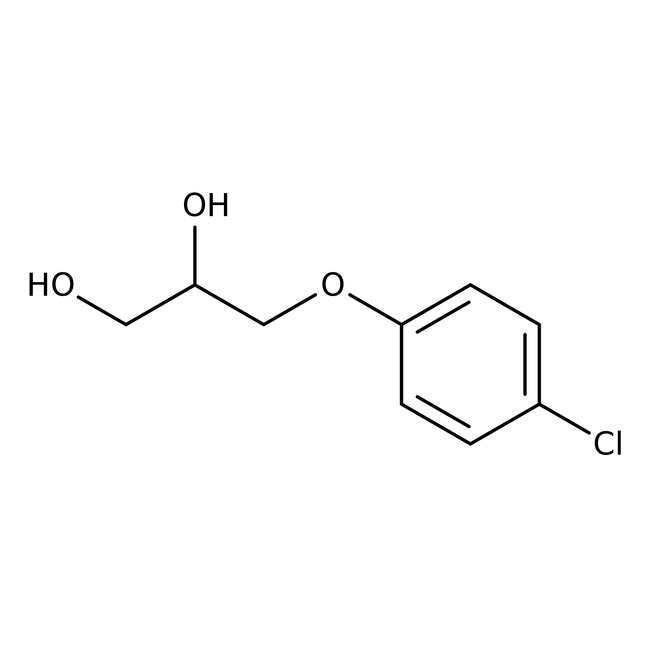
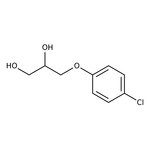
3-(4-Chlorophenoxy)-1,2-propanediol, 99%
CAS: 104-29-0 | C9H11ClO3 | 202.634 g/mol
Catalog number ALFA17386.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material3-(4-Chlorophenoxy)-1,2-propanediol
Melting Point78°C to 82°C
CAS104-29-0
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
It inhibits IgE-mediated histamine release. 3-(4-Chlorophenoxy)-1,2-propanediol is also used as an antimycotic agent. It is a centrally acting muscle relaxant.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It inhibits IgE-mediated histamine release. 3-(4-Chlorophenoxy)-1,2-propanediol is also used as an antimycotic agent. It is a centrally acting muscle relaxant.
Solubility
Slightly soluble in water.
Notes
Store at 78-82°C. Keep away from strong oxidizing agents.
It inhibits IgE-mediated histamine release. 3-(4-Chlorophenoxy)-1,2-propanediol is also used as an antimycotic agent. It is a centrally acting muscle relaxant.
Solubility
Slightly soluble in water.
Notes
Store at 78-82°C. Keep away from strong oxidizing agents.
RUO – Research Use Only
General References:
- James P. Collman.; James A. Belmont.; John I. Brauman. A silica-supported rhodium hydroformylation catalyst: evidence for dinuclear elimination. J. Am. Chem. Soc. 1983, 105 (25), 7288-7294.
- Jing Quan, Qi Wu.; Xian-Fu Lin. Synthesis of polymeric prodrugs of chlorphenesin with saccharide branches by chemo-enzymatic regioselective strategy. Polymer. 2007, 48 (9),2595-2604.