Search Thermo Fisher Scientific
Thermo Scientific Chemicals
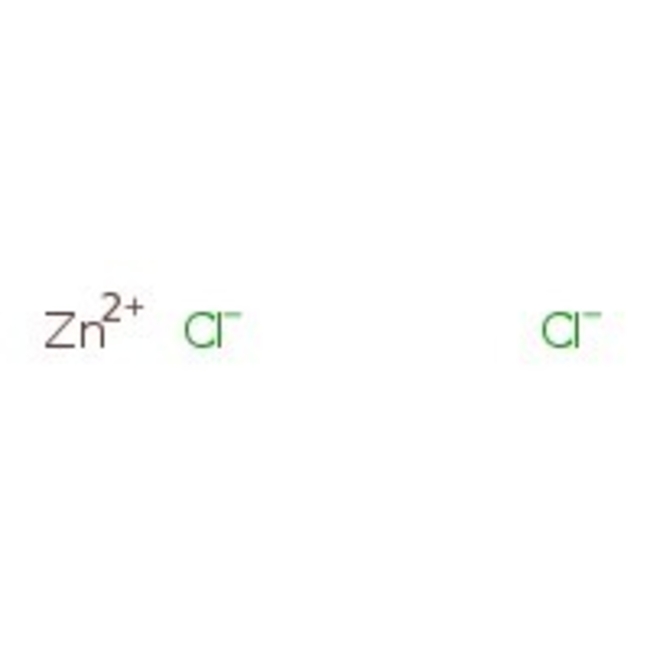
Zinc chloride, anhydrous, 98+%
Zinc chloride, >-98%, Cl2Zn, CAS Number-7646-85-7, zinkchloride, zinc ii chloride, zintrace, zinc dichloride, zinc chloride zncl2, zinc chloride, anhydrous, zine dichloride, zinc chloride fume, zinc chloride, zinc butter, 500g, 732 deg.C, CHEBI:49976, 2.91 | CAS: 7646-85-7 | Cl2Zn | 136.28 g/mol
Catalog number ALFA16281.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Boiling Point732°C
Chemical Name or MaterialZinc chloride
Melting Point290°C
Name Noteanhydrous
CAS7646-85-7
View more
Zinc chloride is used as a catalyst, dehydrating and condensing agent, soldering flux, and metal etchant. It is also employed in preserving anatomical specimens, wood preservatives, deodorant, disinfecting and embalming materials. It is likewise employed as a mordant in printing and dyeing materials and in the vulcanizing processes of fiber and rubber. It is useful as an electrolyte in dry cell batteries, in metal industry, in galvanizing iron and as an electrolyte for electroplating. It is useful in organic synthesis, for example, in the Friedel-Crafts acylatoin, Fisher indole synthesis, Lucas reagent (zinc chloride with HCl), activation of allylic/benzylic halides towards reaction with olefins or with sodium cyanoborohydride catalyzed reduction of halides, organozinc reagents for use in Negishi coupling, and aldol condensation reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Zinc chloride is used as a catalyst, dehydrating and condensing agent, soldering flux, and metal etchant. It is also employed in preserving anatomical specimens, wood preservatives, deodorant, disinfecting and embalming materials. It is likewise employed as a mordant in printing and dyeing materials and in the vulcanizing processes of fiber and rubber. It is useful as an electrolyte in dry cell batteries, in metal industry, in galvanizing iron and as an electrolyte for electroplating. It is useful in organic synthesis, for example, in the Friedel-Crafts acylatoin, Fisher indole synthesis, Lucas reagent (zinc chloride with HCl), activation of allylic/benzylic halides towards reaction with olefins or with sodium cyanoborohydride catalyzed reduction of halides, organozinc reagents for use in Negishi coupling, and aldol condensation reactions.
Solubility
Soluble in water, dilute HCl, alcohol, glycerol, acetone and ether.
Notes
Hygroscopic.
Zinc chloride is used as a catalyst, dehydrating and condensing agent, soldering flux, and metal etchant. It is also employed in preserving anatomical specimens, wood preservatives, deodorant, disinfecting and embalming materials. It is likewise employed as a mordant in printing and dyeing materials and in the vulcanizing processes of fiber and rubber. It is useful as an electrolyte in dry cell batteries, in metal industry, in galvanizing iron and as an electrolyte for electroplating. It is useful in organic synthesis, for example, in the Friedel-Crafts acylatoin, Fisher indole synthesis, Lucas reagent (zinc chloride with HCl), activation of allylic/benzylic halides towards reaction with olefins or with sodium cyanoborohydride catalyzed reduction of halides, organozinc reagents for use in Negishi coupling, and aldol condensation reactions.
Solubility
Soluble in water, dilute HCl, alcohol, glycerol, acetone and ether.
Notes
Hygroscopic.
RUO – Research Use Only
General References:
- Salyulev, A. B.; Potapov, A. M. Conductivity of Some Molten Chlorides at Elevated Temperatures I. Experimental and Calculation Techniques for BeCl2, ZnCl2, and PbCl2. J. Chem. Eng. Data. 2015, 60 (3), 484-492.
- Kamandari, H.; Rafsanjani, H. H.; Najjarzadeh, H.; Eksiri, Z. Influence of process variables on chemically activated carbon from pistachio shell with ZnCl2 and KOH Journal on Chemical Intermediates. 2015, 41 (1), 71-81.

.png-150.jpg)
