Search Thermo Fisher Scientific
Thermo Scientific Chemicals
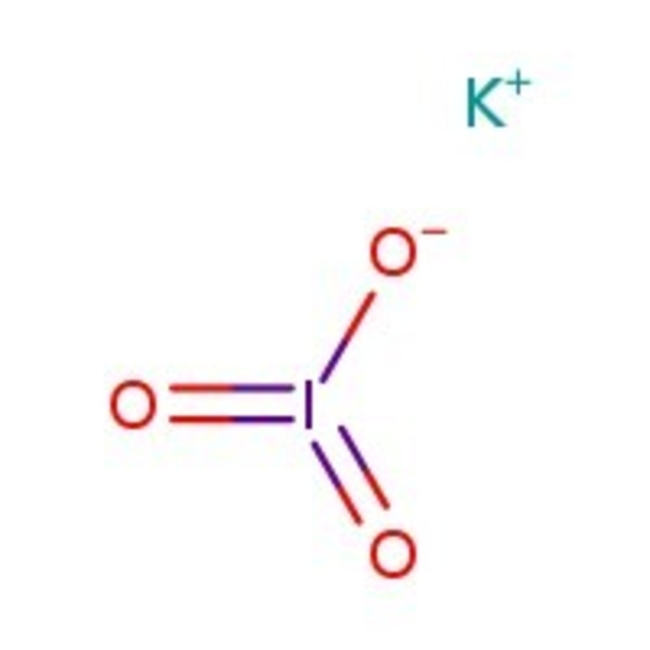
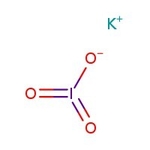
Potassium iodate, 98%
Potassium iodate, 98%, IKO3, CAS Number-7758-05-6, iodic acid, potassium salt, kaliumjodat, epa pesticide chemical code 075703, iodic acid hio3 , potassium salt 1:1, potassium iodate, iodic acid hio3 , potassium salt, caswell no. 693a, potassium triodate, potassium iodine oxide kio3, unii-i139e44nhl | CAS: 7758-05-6 | IKO3 | 214.00 g/mol
Catalog number ALFA16162.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialPotassium iodate
Melting Point560°C (decomposition)
CAS7758-05-6
Health Hazard 1H272-H302-H315-H319-H335
Health Hazard 2GHS H Statement
H272-H315-H319-H335
May intensify fire; oxidizer.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H272-H315-H319-H335
May intensify fire; oxidizer.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
Potassium iodate is an oxidizing agent used to prepare 2-styrylchromones from o-hydroxy-ω-cinnamylideneacetophenones by oxidative cyclization. It is used to iodinate table salt in order to prevent iodine deficiency. It is an active ingredient in baby formula milk. As a maturing agent, it is used in baking. Furthermore, it is involved in the protection of radioactive iodine accumulation in the thyroid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium iodate is an oxidizing agent used to prepare 2-styrylchromones from o-hydroxy-ω-cinnamylideneacetophenones by oxidative cyclization. It is used to iodinate table salt in order to prevent iodine deficiency. It is an active ingredient in baby formula milk. As a maturing agent, it is used in baking. Furthermore, it is involved in the protection of radioactive iodine accumulation in the thyroid.
Solubility
Soluble in water, potassium iodide solution. Insoluble in alcohol, liquid ammonia and nitric acid.
Notes
Incompatible with aluminum, strong oxidizing agents, sulfides, peroxides, metals and reducing agents. Avoid heat, shock and friction.
Potassium iodate is an oxidizing agent used to prepare 2-styrylchromones from o-hydroxy-ω-cinnamylideneacetophenones by oxidative cyclization. It is used to iodinate table salt in order to prevent iodine deficiency. It is an active ingredient in baby formula milk. As a maturing agent, it is used in baking. Furthermore, it is involved in the protection of radioactive iodine accumulation in the thyroid.
Solubility
Soluble in water, potassium iodide solution. Insoluble in alcohol, liquid ammonia and nitric acid.
Notes
Incompatible with aluminum, strong oxidizing agents, sulfides, peroxides, metals and reducing agents. Avoid heat, shock and friction.
RUO – Research Use Only
General References:
- Tang, Y.; Li, Y.; Yu, Z.; Bai, Y.; Chen, Y.; Sun, Y.; Wan, P. Energy-saving synthesis of potassium iodate via electrolysis of potassium iodine and O2 in a membraneless cell. Green Chem. 2012, 14 (2), 334-337.
- Muraleedharan, K. Thermal decomposition kinetics of potassium iodate. J. Therm. Anal. Calorim. 2011, 103 (3), 943-955.