Search Thermo Fisher Scientific
Thermo Scientific Chemicals
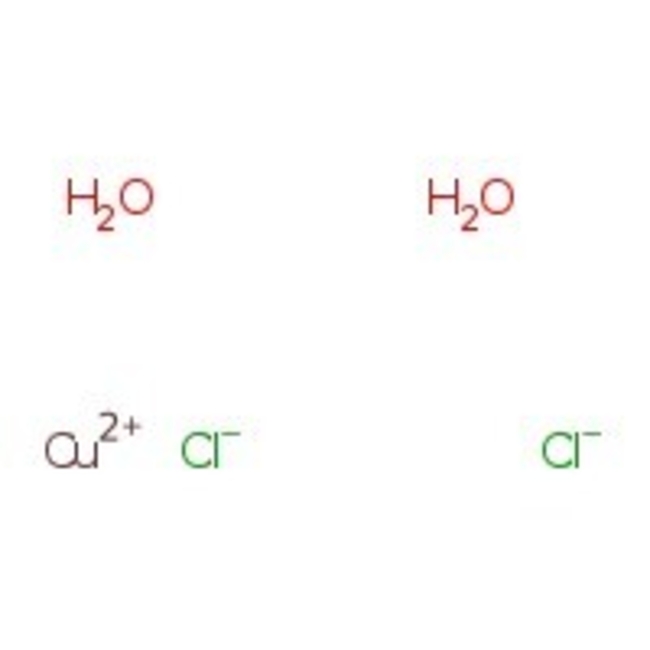
Copper(II) chloride dihydrate, 99%
CAS: 10125-13-0 | Cl2CuH4O2 | 170.48 g/mol
Catalog number ALFA16064.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialCopper(II) chloride dihydrate
Melting Point100°C (decomposition)
CAS10125-13-0
Health Hazard 1H302+H312-H315-H318
Health Hazard 2GHS H Statement
H314-H318-H302
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
H314-H318-H302
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
View more
Copper(II) chloride is used as a catalyst for organic and inorganic reactions, mordant for dyeing and printing materials, pigment for glass, ceramics, as wood preservative, as disinfectant, and as a catalyst in the output of chlorine from hydrogen chloride. It is likewise employed in the oil industry as a purifying agent, in the manufacture of laundry marking inks, in metallurgy to recover mercury from ores, in refining copper, silver and gold, in tinting baths of iron and tin, in photography, in pyrotechnics, and to get rid of lead compounds from gasoline and oils.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Copper(II) chloride is used as a catalyst for organic and inorganic reactions, mordant for dyeing and printing materials, pigment for glass, ceramics, as wood preservative, as disinfectant, and as a catalyst in the output of chlorine from hydrogen chloride. It is likewise employed in the oil industry as a purifying agent, in the manufacture of laundry marking inks, in metallurgy to recover mercury from ores, in refining copper, silver and gold, in tinting baths of iron and tin, in photography, in pyrotechnics, and to get rid of lead compounds from gasoline and oils.
Solubility
Soluble in water, methanol and ethanol.
Notes
Incompatible with materials such as oxidizing agents, metals and acids.
Copper(II) chloride is used as a catalyst for organic and inorganic reactions, mordant for dyeing and printing materials, pigment for glass, ceramics, as wood preservative, as disinfectant, and as a catalyst in the output of chlorine from hydrogen chloride. It is likewise employed in the oil industry as a purifying agent, in the manufacture of laundry marking inks, in metallurgy to recover mercury from ores, in refining copper, silver and gold, in tinting baths of iron and tin, in photography, in pyrotechnics, and to get rid of lead compounds from gasoline and oils.
Solubility
Soluble in water, methanol and ethanol.
Notes
Incompatible with materials such as oxidizing agents, metals and acids.
RUO – Research Use Only
General References:
- Bokhimi, X.; Morales, A.; Novaro, O. Effect of Copper Precursor on the Stabilization of Titania Phases, and the Optical Properties of Cu/TiO2 Prepared with the Sol-Gel Technique. Chem. Mater. 1997, 9 (11), 2616-2620.
- Azzaroni, O.; Cipollone, M.; Vela, M.E.; Salvarezza, R.C. Protective Properties of Dodecanethiol Layers on Copper Surfaces: The Effect of Chloride Anions in Aqueous Environments. Langmuir 2001, 17 (5), 1483-1487.