Search Thermo Fisher Scientific
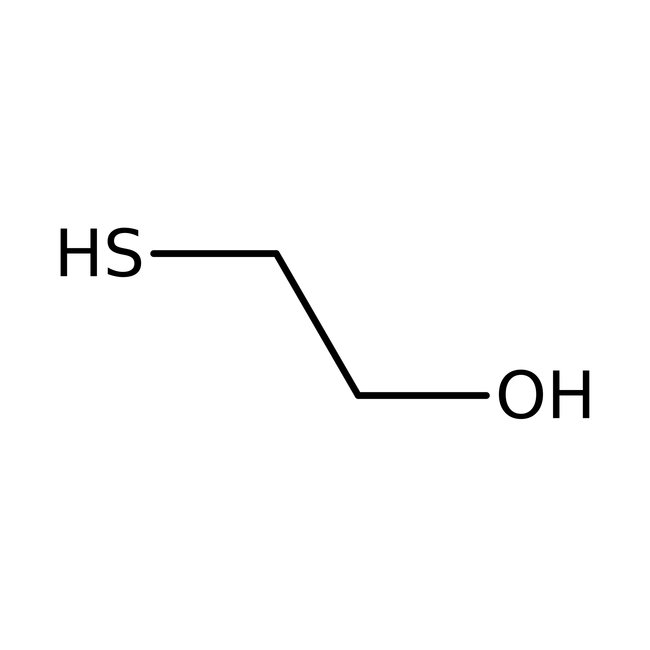
2-Mercaptoethanol, 98+%
H301-H310-H318-H227-H315-H317-H335
Toxic if swallowed.
Fatal in contact with skin.
Causes serious eye damage.
Combustible liquid.
Causes skin irritation.
May cause an allergic skin reaction.
May cause respiratory irritation.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
• 2-Mercaptoethanol is a reducing agent that can be used in studies to solubilize proteins by reducing disulfide linkages
Application
• 2-Mercaptoethanol acts as a reducing agent in electrophoresis, amino acid detection, and distinguishing ssDNA/dsDNA
• It can also be used as a standard buffer
• It can also be used in some RNA isolation procedures to eliminate ribonuclease
• In biochemistry, it is useful for studying the activity of the immune system
• Furthermore, it can be used in laboratory applications involving preparation of multifunctional polymeric micelles and nano-graphene for cellular imaging
General References:
- Protective agent for preventing oxidation of thiol groups in proteins: Enzyme Assays, R. Eisenthal and M. J. Danson, Eds., OUP, Oxford (1992), p 265.
- In the presence of BF3 etherate, reacts with carbonyl compounds to give monothioacetals (1,3-oxathiolanes): J. Org. Chem., 33, 2133 (1968). Amberlyst 15 is also effective: Synthesis, 1826 (2001), as is In(OTf)3: Synlett, 1535 (2002). 1,3-Oxathiolanes can be prepared from acid-sensitive aldehydes with LiBH4 as catalyst in acetonitrile: Synlett, 238 (2001). Cleavage can be effected, e.g. with chloramine-T: Tetrahedron Lett., 3445 (1971), isoamyl nitrite: Tetrahedron. Lett., 3561 (1978), or periodic acid: Tetrahedron Lett., 37, 4331 (1996). Selective cleavage of oxathiolanes in the presence of dithiolanes has been achieved with triphenylcarbenium tetrafluoroborate: J. Chem. Soc., Perkin 1, 542 (1972), or 4-nitrobenzaldehyde catalyzed by TMS-OTf: J. Chem. Soc. Chem. Commun., 1937 (1994). Compare 1,2-Ethanedithiol, L12865.
- 2-Mercaptoethanol is a reagent for the specific cleavage of the N-dithiasuccinimide protecting group from amines. See Chlorocarbonyl¬sulfenyl¬ chloride, L06432.
- Sugimoto, Y.; Kitazumi, Y.; Shirai, O.; Yamamoto, M.; Kano, K. Role of 2-mercaptoethanol in direct electron transfer-type bioelectrocatalysis of fructose dehydrogenase at Au electrodes. Electrochim. Acta 2015, 170, 242-247.
- Mohebbi, S.; Eslami, S. Electrocatalytic oxidation of 2-mercaptoethanol using modified glassy carbon electrode by MWCNT in combination with unsymmetrical manganese(II) Schiff base complexes. Mater. Res. Bull. 2015, 66, 219-225.