Search Thermo Fisher Scientific
Thermo Scientific Chemicals
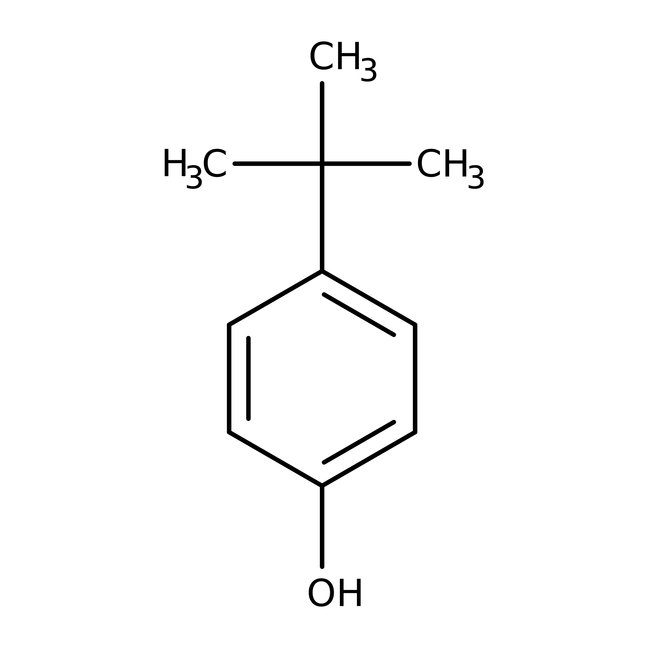
4-tert-Butylphenol, 99%
CAS: 98-54-4 | C10H14O | 150.221 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15871.36 | 500 g |
Catalog number ALFA15871.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material4-tert-Butylphenol
CAS98-54-4
Health Hazard 1H315-H318-H361f
Health Hazard 2GHS H Statement
H318-H317-H335-H315
H318-H317-H335-H315
Health Hazard 3P201-P202-P264b-P281-P302+P352-P305+P351+P338-P308+P313-P310-P332+P313-P362-P501c
View more
4-tert-butylphenol on condensation with formaldehyde gives calix[5]arene which is used in enzyme mimetics.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-tert-butylphenol on condensation with formaldehyde gives calix[5]arene which is used in enzyme mimetics.
Solubility
Soluble in water. [8.7 g/L (20°C)]
Notes
Store at room temperature. Keep away from oxidizing agents and acids.
4-tert-butylphenol on condensation with formaldehyde gives calix[5]arene which is used in enzyme mimetics.
Solubility
Soluble in water. [8.7 g/L (20°C)]
Notes
Store at room temperature. Keep away from oxidizing agents and acids.
RUO – Research Use Only
General References:
- C. David Gutsche.; Balram Dhawan.; Kwang Hyun No.; Ramamurthi Muthukrishnan. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J. Am. Chem. Soc. 1981, 103 (13),3782-3792 .
- The acid-catalyzed removal of t-butyl groups from phenols (reverse Friedel-Crafts reaction), can be brought about without the need for t-butyl acceptors by using aluminum chloride as catalyst in dichloromethane at ambient temperature: Synth. Commun., 18, 1783 (1988).