Search Thermo Fisher Scientific
Thermo Scientific Chemicals
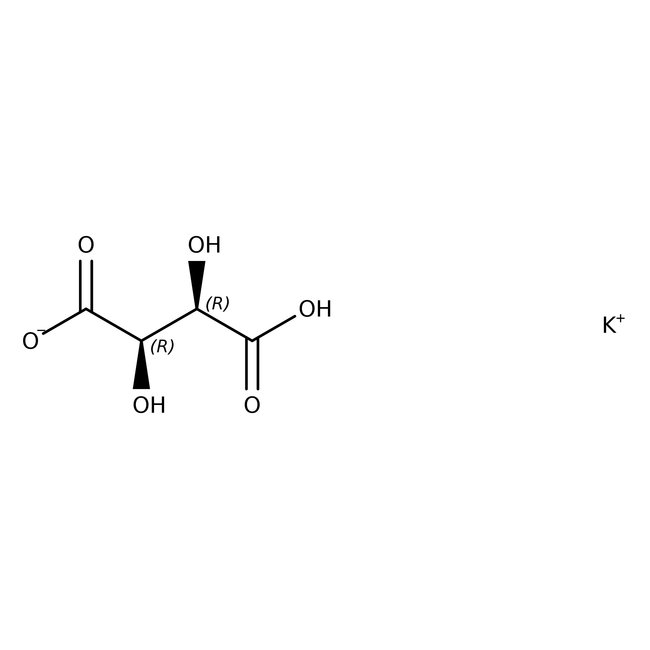
Potassium hydrogen L-tartrate, 98+%
CAS: 868-14-4 | C4H5KO6 | 188.18 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15281.30 | 250 g |
Catalog number ALFA15281.30
Price (MYR)
223.00
EA
Quantity:
250 g
Price (MYR)
223.00
EA
Specifications
Chemical Name or MaterialPotassium hydrogen L-tartrate
CAS868-14-4
Melting Point∼267°C (decomposition)
Recommended StorageAmbient temperatures
Density1.954
View more
Potassium hydrogen L-tartrate is used for stabilizing egg whites, whipped cream and for anti-caking and thickening. It is utilized for the prevention of crystallization of sugar syrups and reduces the discoloration of boiled vegetables. It is an active component in baking powder. It is used in household items in association with lemon juice or white vinegar to clean metals such as brass, aluminum and copper. Furthermore, it serves as a primary reference standard for a pH buffer.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium hydrogen L-tartrate is used for stabilizing egg whites, whipped cream and for anti-caking and thickening. It is utilized for the prevention of crystallization of sugar syrups and reduces the discoloration of boiled vegetables. It is an active component in baking powder. It is used in household items in association with lemon juice or white vinegar to clean metals such as brass, aluminum and copper. Furthermore, it serves as a primary reference standard for a pH buffer.
Solubility
Soluble in water and dilute mineral acid. Insoluble in alcohol.
Notes
Incompatible with strong oxidizing agents.
Potassium hydrogen L-tartrate is used for stabilizing egg whites, whipped cream and for anti-caking and thickening. It is utilized for the prevention of crystallization of sugar syrups and reduces the discoloration of boiled vegetables. It is an active component in baking powder. It is used in household items in association with lemon juice or white vinegar to clean metals such as brass, aluminum and copper. Furthermore, it serves as a primary reference standard for a pH buffer.
Solubility
Soluble in water and dilute mineral acid. Insoluble in alcohol.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Barril, C.; Clark, A. C.; Scollary, G. R. Chemistry of ascorbic acid and sulfur dioxide as an antioxidant system relevant to white wine. Anal. Chim. Acta 2012, 732, 186-193.
- Clark, A. C.; Dias, D. A.; Smith, T. A.; Ghiggino, K. P.; Scollary, G. R. Iron(III) tartrate as a potential precursor of light-induced oxidative degradation of white wine: Studies in a model wine system. J. Agric. Food. Chem. 2011, 59 (8), 3575-3581.