Search Thermo Fisher Scientific
Thermo Scientific Chemicals
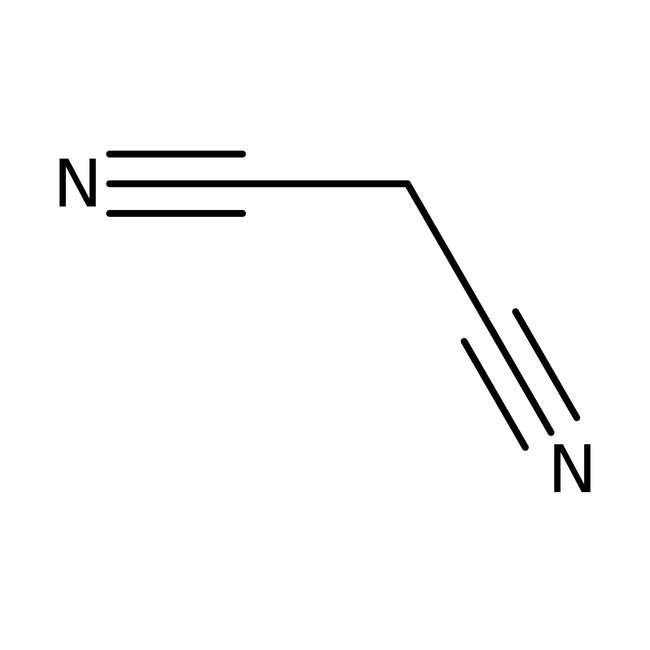
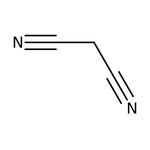
Malononitrile, 99%
CAS: 109-77-3 | C3H2N2 | 66.063 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15046.22 | 100 g |
Catalog number ALFA15046.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialMalononitrile
CAS109-77-3
Health Hazard 1H301+H311+H331
Health Hazard 2GHS H Statement
H301-H311-H331
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
H301-H311-H331
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P311-P312-P330-P361-P363-P501c
View more
Crosslinking agent for modification of proteins via amidationMalononitrile is used as a precursor in the preparation of anti-cancer drug, triamterene, adenine, methotrexate, thiamine, acrylic fibers and dyes. It is used in Knoevenagel condensation to prepare 2-chlorobenzalmalononitrile by reacting with 2-chlorobenzaldehyde. It acts as a leaching agent for gold. It is used as a raw material for the Gewald reaction of ketone or aldehyde to produce 2-aminothiophene using elemental sulfur and a base. Further, it plays an important role in the preparation of [1,6]-naphthyridines, gamma-ketoamides, 1,4-dihydropyridine, chromeno[2,3-b]pyridine and dihydro-1,4-dithiepine frameworks.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Crosslinking agent for modification of proteins via amidationMalononitrile is used as a precursor in the preparation of anti-cancer drug, triamterene, adenine, methotrexate, thiamine, acrylic fibers and dyes. It is used in Knoevenagel condensation to prepare 2-chlorobenzalmalononitrile by reacting with 2-chlorobenzaldehyde. It acts as a leaching agent for gold. It is used as a raw material for the Gewald reaction of ketone or aldehyde to produce 2-aminothiophene using elemental sulfur and a base. Further, it plays an important role in the preparation of [1,6]-naphthyridines, gamma-ketoamides, 1,4-dihydropyridine, chromeno[2,3-b]pyridine and dihydro-1,4-dithiepine frameworks.
Solubility
Soluble in water, acetone, acetic acid, chloroform, ethanol, ether and benzene.
Notes
Store in a cool place. Incompatible with strong oxidizing agents, strong reducing agents, strong acids and strong bases.
Crosslinking agent for modification of proteins via amidationMalononitrile is used as a precursor in the preparation of anti-cancer drug, triamterene, adenine, methotrexate, thiamine, acrylic fibers and dyes. It is used in Knoevenagel condensation to prepare 2-chlorobenzalmalononitrile by reacting with 2-chlorobenzaldehyde. It acts as a leaching agent for gold. It is used as a raw material for the Gewald reaction of ketone or aldehyde to produce 2-aminothiophene using elemental sulfur and a base. Further, it plays an important role in the preparation of [1,6]-naphthyridines, gamma-ketoamides, 1,4-dihydropyridine, chromeno[2,3-b]pyridine and dihydro-1,4-dithiepine frameworks.
Solubility
Soluble in water, acetone, acetic acid, chloroform, ethanol, ether and benzene.
Notes
Store in a cool place. Incompatible with strong oxidizing agents, strong reducing agents, strong acids and strong bases.
RUO – Research Use Only
General References:
- The Na derivative can be arylated by reaction with aryl halides with a Pd catalyst: J. Chem. Soc., Chem. Commun., 932 (1984). In the presence of butadiene, an additional 4-carbon unit is introduced: J. Chem. Soc., Perkin 1, 647 (1990):
- Ternary condensation with an aromatic aldehyde and a cycloalkanone in the presence of ammonium acetate can give a variety of products. With cyclohexanone, the 2-amino-4-aryl-5,6,7,8-tetrahydroquinoline-3-carbonitriles are the major products: J. Chem. Res. (Synop.), 146 (1995).
- Reviews of the chemistry of malononitrile: Chem. Rev., 69, 591 (1969); Synthesis, 165, 241 (1978); 925 (1981); Synlett, 2247 (2004).
- Caruana, L.; Mondatori, M.; Corti, V.; Morales, S.; Mazzanti, A.; Fochi, M.; Bernardi, L. Catalytic Asymmetric Addition of Meldrum’s Acid, Malononitrile, and 1,3-Dicarbonyls to ortho-Quinone Methides Generated In Situ Under Basic Conditions. Chem. Eur. J. 2015, 21 (16), 6037-6041.
- Adili, A.; Tao, Z. L.; Chen, D. F.; Han, Z. Y. Quinine-catalyzed highly enantioselective cycloannulation of o-quinone methides with malononitrile. Org. Biomol. Chem. 2015, 13 (8), 2247-2250.