Search Thermo Fisher Scientific
Thermo Scientific Chemicals
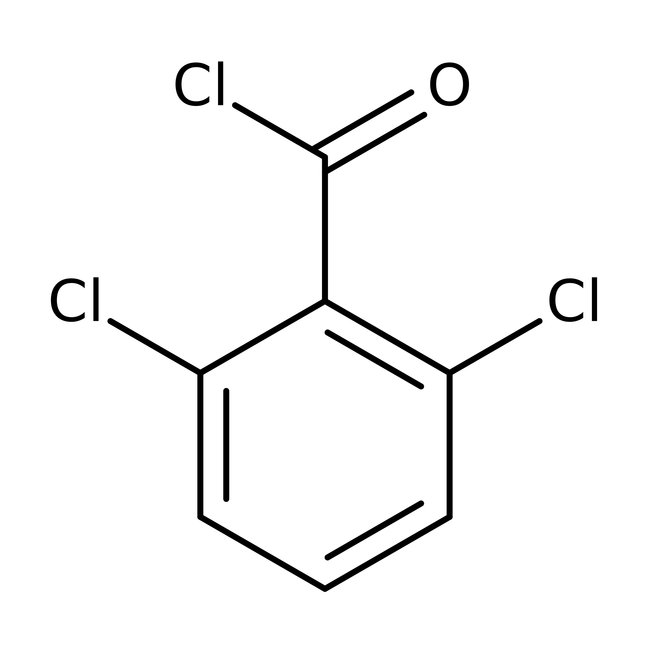
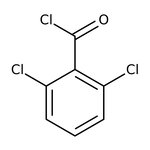
2,6-Dichlorobenzoyl chloride, 98%
CAS: 4659-45-4 | C7H3Cl3O | 209.45 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14839.09 | 10 g |
Catalog number ALFA14839.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Specifications
Chemical Name or Material2,6-Dichlorobenzoyl chloride
CAS4659-45-4
Health Hazard 1H314
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
2,6-Dichlorobenzoyl chloride acts as an important intermediate in organic synthesis, pharmaceuticals, agrochemicals and dyestuffs. It is employed in the substrate activity screening method for the rapid development of novel substrates and their conversion into non-peptidic inhibitors of Cys and Ser proteases. It is also used in the preparation of 1-acyliridoles and (-)-aspicilin. In addition to this, it undergoes esterification of (fluoren-9-ylmethoxy)carbonyl (Fmoc)-amino acids to 4-alkoxybenzyl alcohol polystyrene.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,6-Dichlorobenzoyl chloride acts as an important intermediate in organic synthesis, pharmaceuticals, agrochemicals and dyestuffs. It is employed in the substrate activity screening method for the rapid development of novel substrates and their conversion into non-peptidic inhibitors of Cys and Ser proteases. It is also used in the preparation of 1-acyliridoles and (-)-aspicilin. In addition to this, it undergoes esterification of (fluoren-9-ylmethoxy)carbonyl (Fmoc)-amino acids to 4-alkoxybenzyl alcohol polystyrene.
Solubility
Miscible with alcohol, ether and acetone. Slightly miscible with heptane. Immiscible with water.
Notes
Moisture sensitive. Incompatible with alcohols, oxidizing agents and strong bases.
2,6-Dichlorobenzoyl chloride acts as an important intermediate in organic synthesis, pharmaceuticals, agrochemicals and dyestuffs. It is employed in the substrate activity screening method for the rapid development of novel substrates and their conversion into non-peptidic inhibitors of Cys and Ser proteases. It is also used in the preparation of 1-acyliridoles and (-)-aspicilin. In addition to this, it undergoes esterification of (fluoren-9-ylmethoxy)carbonyl (Fmoc)-amino acids to 4-alkoxybenzyl alcohol polystyrene.
Solubility
Miscible with alcohol, ether and acetone. Slightly miscible with heptane. Immiscible with water.
Notes
Moisture sensitive. Incompatible with alcohols, oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Furse, S.; Mak, L.; Tate, E. W.; Templer, R. H.; Ces, O.; Woscholski, R.; Gaffney, P. R. Synthesis of unsaturated phosphatidylinositol 4-phosphates and the effects of substrate unsaturation on SopB phosphatase activity. Org. Biomol. Chem. 2015, 13 (7), 2001-2011.
- Zhang, T.; Paluch, K.; Scalabrino, G.; Frankish, N.; Healy, A. M.; Sheridan, H. Molecular structure studies of (1S,2S)-2-benzyl-2,3-dihydro-2-(1H-inden-2-yl)-1H-inden-1-ol. J. Mol. Struct. 2015, 1083, 286-299.