Search Thermo Fisher Scientific
Thermo Scientific Chemicals
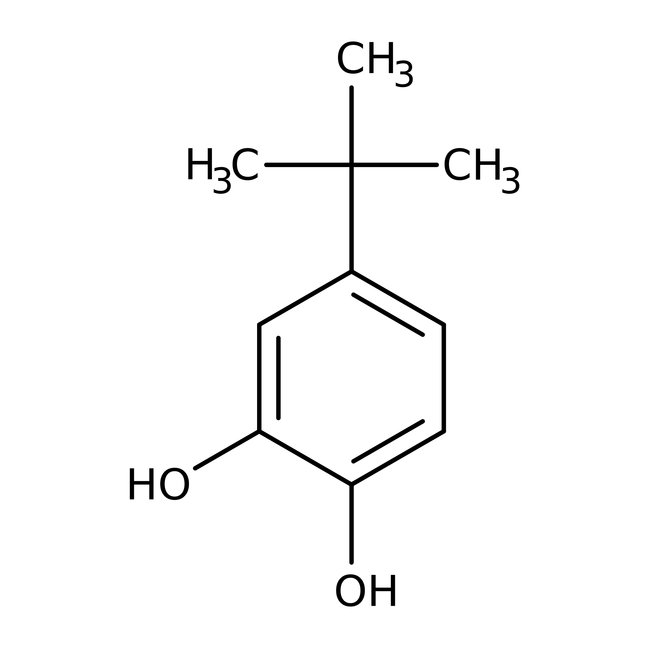
4-tert-Butylcatechol, 98%
CAS: 98-29-3 | C10H14O2 | 166.22 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14599.30 | 250 g |
Catalog number ALFA14599.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material4-tert-Butylcatechol
CAS98-29-3
Health Hazard 1H302+H312-H314-H317-H335-H350
Health Hazard 2GHS H Statement
H311-H314-H318-H317
Toxic in contact with skin.
Causes severe skin burns and eye damage.
Causes serious eye damage.
May cause an allergic skin reaction.
H311-H314-H318-H317
Toxic in contact with skin.
Causes severe skin burns and eye damage.
Causes serious eye damage.
May cause an allergic skin reaction.
Health Hazard 3P201-P202-P260-P264b-P270-P271-P272-P280g-P281-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P333+P313-P363-P501c
View more
4-tert-Butylcatechol is widely utilized as an inhibitor in polymerization of butadiene, styrene, vinyl acetate and other reactive monomers. It plays an important role in the synthesis of tungsten oxide nanoparticles by nonaqueous sol-gel process. It acts as a stabilizer in the manufacturing of polyurethane foam. It is employed as an antioxidant for synthetic rubber, polymers and oil derivatives. It is also utilized as a purification agent for aminoformate catalysts.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-tert-Butylcatechol is widely utilized as an inhibitor in polymerization of butadiene, styrene, vinyl acetate and other reactive monomers. It plays an important role in the synthesis of tungsten oxide nanoparticles by nonaqueous sol-gel process. It acts as a stabilizer in the manufacturing of polyurethane foam. It is employed as an antioxidant for synthetic rubber, polymers and oil derivatives. It is also utilized as a purification agent for aminoformate catalysts.
Solubility
Soluble in ether, acetone, alcohol, and methanol. Insoluble in water.
Notes
Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
4-tert-Butylcatechol is widely utilized as an inhibitor in polymerization of butadiene, styrene, vinyl acetate and other reactive monomers. It plays an important role in the synthesis of tungsten oxide nanoparticles by nonaqueous sol-gel process. It acts as a stabilizer in the manufacturing of polyurethane foam. It is employed as an antioxidant for synthetic rubber, polymers and oil derivatives. It is also utilized as a purification agent for aminoformate catalysts.
Solubility
Soluble in ether, acetone, alcohol, and methanol. Insoluble in water.
Notes
Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Radical inhibitor and antioxidant.
- Rafiee, M. Electrochemical oxidation of 4-tert-butylcatechol in the presence of β-cyclodextrin: Interplay between E and CE mechanisms. Int. J. Chem. Kinet. 2012, 44 (8), 507-513.
- Nematollahi, D.; Rafiee, M.; Samadi-Maybodi, A. Mechanistic study of electrochemical oxidation of 4-tert-butylcatechol: A facile electrochemical method for the synthesis of new trimer of 4-tert-butylcatechol. Electrochim. Acta 2004, 49 (15), 2495-2502.