Search Thermo Fisher Scientific
Thermo Scientific Chemicals
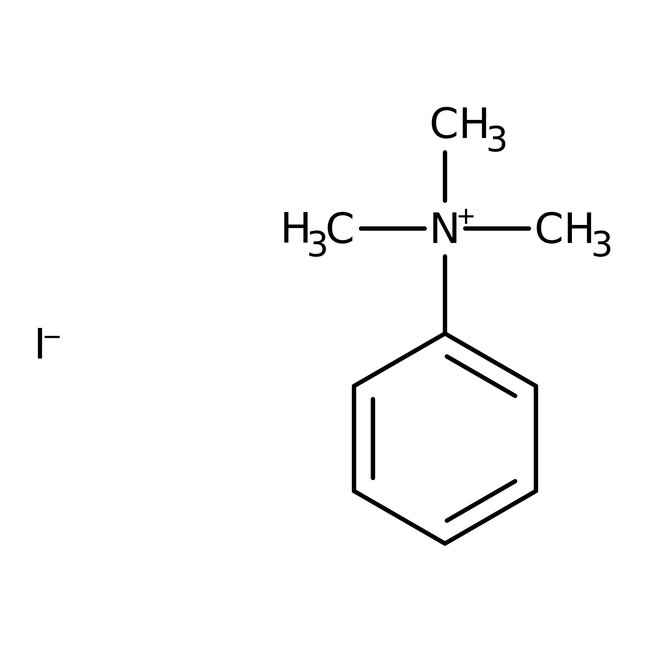
Phenyltrimethylammonium iodide, 99%
CAS: 98-04-4 | C9H14IN | 263.122 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14402.22 | 100 g |
Catalog number ALFA14402.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialPhenyltrimethylammonium iodide
CAS98-04-4
Health Hazard 1H315-H318-H335
Health Hazard 2GHS H Statement
H311-H302-H315-H319-H335
Toxic in contact with skin.
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H311-H302-H315-H319-H335
Toxic in contact with skin.
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P310-P312-P332+P313-P362-P501c
View more
Phenyltrimethylammonium iodide is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenyltrimethylammonium iodide is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
Solubility
Soluble in water and methanol.
Notes
Store at room temperature. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Light sensitive. It is hygroscopic in nature. Incompatible with strong oxidizing agents.
Phenyltrimethylammonium iodide is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
Solubility
Soluble in water and methanol.
Notes
Store at room temperature. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Light sensitive. It is hygroscopic in nature. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Robert O. Hutchins.; Duraisamy Kandasamy.; Frank DuxIII.; Cynthia A. Maryanoff.; David Rotstein.; Barry Goldsmith.; William Burgoyne.; Frank Cistone.; Joseph Dalessandro.; Joseph Puglis. Nucleophilic borohydride: selective reductive displacement of halides, sulfonate esters, tertiary amines, and N,N-disulfonimides with borohydride reagents in polar aprotic solvents. J. Org. Chem. 1978, 43 (11), 2259-2267.
- A. Pass, A.I.C.; A. M. Ward, D.Sc., A.I.C. The determination of cadmium in the presence of zinc, in spelter and in zinc ores. Analyst. 1933, 58 667-672.
- Reagent for Cd.