Search Thermo Fisher Scientific
Thermo Scientific Chemicals
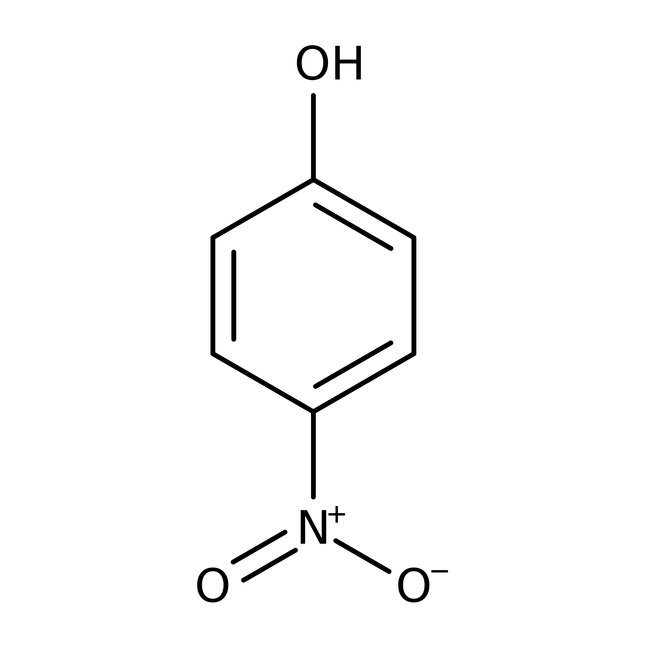
4-Nitrophenol, 99%
CAS: 100-02-7 | C6H5NO3 | 139.11 g/mol
Catalog number ALFA14376.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material4-Nitrophenol
CAS100-02-7
Health Hazard 1H301-H312+H332-H373-H500
Health Hazard 2GHS H Statement
H373-H302-H312-H332
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
H373-H302-H312-H332
May cause damage to organs through prolonged or repeated exposure.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Health Hazard 3P260-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P312-P314-P330-P363-P501c
View more
4-Nitrophenol is used as a precursor to prepare phenetidine, acetophenetidne and pH indicator. Its carboxylate ester derivatives are involved as an active component for the construction of amide moieties in peptide synthesis. It is used as an intermediate in the synthesis of paracetamol and N-acetyl-p-aminophenol dyestuffs.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Nitrophenol is used as a precursor to prepare phenetidine, acetophenetidne and pH indicator. Its carboxylate ester derivatives are involved as an active component for the construction of amide moieties in peptide synthesis. It is used as an intermediate in the synthesis of paracetamol and N-acetyl-p-aminophenol dyestuffs.
Solubility
Freely soluble in alcohol, chloroform, ether. Freely soluble in acetone. Slightly soluble in water.
Notes
Incompatible with strong oxidizing agents and strong bases.
4-Nitrophenol is used as a precursor to prepare phenetidine, acetophenetidne and pH indicator. Its carboxylate ester derivatives are involved as an active component for the construction of amide moieties in peptide synthesis. It is used as an intermediate in the synthesis of paracetamol and N-acetyl-p-aminophenol dyestuffs.
Solubility
Freely soluble in alcohol, chloroform, ether. Freely soluble in acetone. Slightly soluble in water.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Acid-base indicator: pH 5.6 - 7.6.
- Forms active esters with N-protected amino acids, useful in peptide synthesis; see, for example: J. Org. Chem., 62, 1356 (1997). For peptide reagents, see Appendix 6.
- Suzuki-type coupling of the triflate with various arylboronic acids gives biaryl derivatives: Tetrahedron, 45, 6679 (1984). For an example of Stille coupling of the triflate with an arylstannane, catalyzed by trans-Dichlorobis(triphenyl phosphine) palladium(II) , 10491, see: Org. Synth. Coll., 9, 553 (1998).
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt-Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4- nitrophenol reduction. Appl. Catal., B 2015, 181, 371-378.
- Chaudhary, S.; Kumar, S.; Mehta, S. K. Glycol modified gadolinium oxide nanoparticles as a potential template for selective and sensitive detection of 4-nitrophenol. J. Mater. Chem. C 2015, 3 (34), 8824-8833.