Search Thermo Fisher Scientific
Thermo Scientific Chemicals
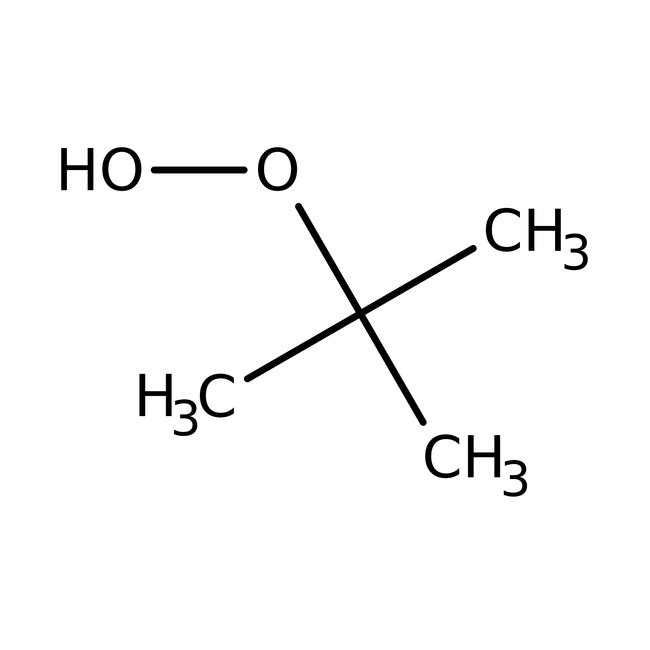
tert-Butyl hydroperoxide, 70% aq. soln.
CAS: 75-91-2 | C4H10O2 | 90.122 g/mol
Catalog number ALFA13926.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or Materialtert-Butyl Hydroperoxide
CAS75-91-2
Health Hazard 1H226-H242-H302-H311-H314-H317-H330-H335-H341
Health Hazard 2GHS H Statement
H300-H310-H330-H314-H226-H242
Fatal if swallowed.
Fatal in contact with skin.
Fatal if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapour.
Heating may cause a fire.
H300-H310-H330-H314-H226-H242
Fatal if swallowed.
Fatal in contact with skin.
Fatal if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapour.
Heating may cause a fire.
Health Hazard 3P201-P202-P210-P220-P233-P234-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P272-P280g-P281-P284-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P333+P313-P363-P370+P37
View more
tert-Butyl hydroperoxide is used as an initiator for radical polymerization and in various oxidation process such as sharpless epoxidation. It is involved in osmium catalyzed vicinal hydroxylation of olefins under alkaline conditions. Furthermore, it is used in catalytic asymmetric oxidation of sulfides to sulfoxides using binaphthol as a chiral auxiliary and in the oxidation of dibenzothiophenes. It plays an important role for the introduction of peroxy groups in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
tert-Butyl hydroperoxide is used as an initiator for radical polymerization and in various oxidation process such as sharpless epoxidation. It is involved in osmium catalyzed vicinal hydroxylation of olefins under alkaline conditions. Furthermore, it is used in catalytic asymmetric oxidation of sulfides to sulfoxides using binaphthol as a chiral auxiliary and in the oxidation of dibenzothiophenes. It plays an important role for the introduction of peroxy groups in organic synthesis.
Solubility
Miscible with water and diethyl ether.
Notes
Light sensitive. Store in cool place. Incompatible with powdered metals, strong oxidizing agents, reducing agents, acids, alkalis and heavy metals.
tert-Butyl hydroperoxide is used as an initiator for radical polymerization and in various oxidation process such as sharpless epoxidation. It is involved in osmium catalyzed vicinal hydroxylation of olefins under alkaline conditions. Furthermore, it is used in catalytic asymmetric oxidation of sulfides to sulfoxides using binaphthol as a chiral auxiliary and in the oxidation of dibenzothiophenes. It plays an important role for the introduction of peroxy groups in organic synthesis.
Solubility
Miscible with water and diethyl ether.
Notes
Light sensitive. Store in cool place. Incompatible with powdered metals, strong oxidizing agents, reducing agents, acids, alkalis and heavy metals.
RUO – Research Use Only
General References:
- Li, D.; Yang, T.; Su, H.; Yu, W. tert-Butyl Hydroperoxide and Tetrabutylammonium Iodide-Promoted Free Radical Cyclization of α-Imino-N-arylamides and alfa-Azido-N-arylamides. Adv. Synth. Catal. 2015, 357 (11), 2529-2539.
- Sousa, C.; Moita, E.; Valentão, P.; Fernandes, F.; Monteiro, P.; Andrade, P. B. Effects of Colored and Noncolored Phenolics of Echium plantagineum L. Bee Pollen in Caco-2 Cells under Oxidative Stress Induced by tert-Butyl Hydroperoxide. J. Agric. Food. Chem. 2015, 63 (7), 2083-2091.