Search Thermo Fisher Scientific
Thermo Scientific Chemicals
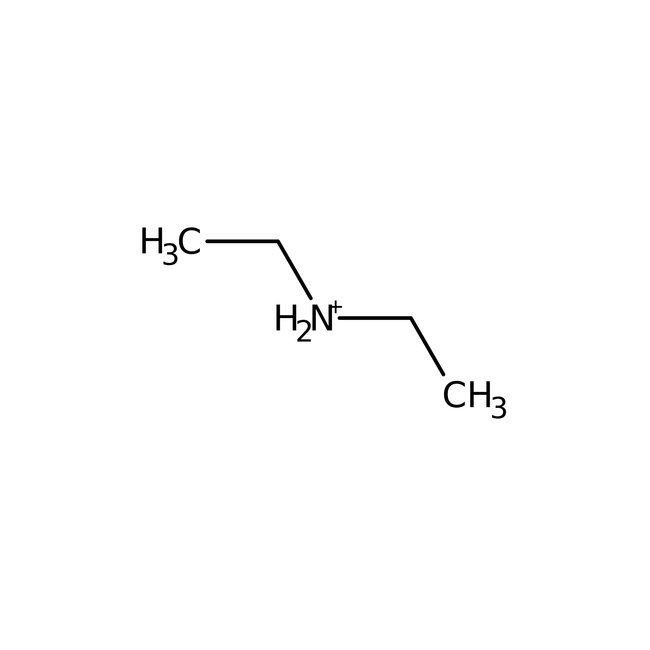
Diethylamine hydrochloride, 99%, Thermo Scientific Chemicals
Catalog number ALFA13637.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialDiethylamine hydrochloride
CAS660-68-4
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
Diethylamine hydrochloride acts as a precursor of atrazine and lysergic acid diethylamide. It is also used as a reactant in the production of dyes and pharmaceutical compounds such as ranitidine. Further, it is used in Mannich reaction with paraformaldehyde. In addition to this, it is employed in the synthesis of diethylaminoethyl (DEAE) cottons by reacting with cotton cellulose in the presence of sodium hydroxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethylamine hydrochloride acts as a precursor of atrazine and lysergic acid diethylamide. It is also used as a reactant in the production of dyes and pharmaceutical compounds such as ranitidine. Further, it is used in Mannich reaction with paraformaldehyde. In addition to this, it is employed in the synthesis of diethylaminoethyl (DEAE) cottons by reacting with cotton cellulose in the presence of sodium hydroxide.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with oxidizing agents.
Diethylamine hydrochloride acts as a precursor of atrazine and lysergic acid diethylamide. It is also used as a reactant in the production of dyes and pharmaceutical compounds such as ranitidine. Further, it is used in Mannich reaction with paraformaldehyde. In addition to this, it is employed in the synthesis of diethylaminoethyl (DEAE) cottons by reacting with cotton cellulose in the presence of sodium hydroxide.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- For Mannich reaction with paraformaldehyde and acetone, see: Org. Synth. Coll., 4, 281 (1963).
- Xiao, J.; Huang, Y.; Song, Z.; Feng, W. Facile catalyst-free synthesis of 2-vinylquinolines via a direct deamination reaction occurring during Mannich synthesis. RSC Adv. 2015, 5 (120), 99095-99098.
- Zavozin, A. G.; Ignat'ev, N. V.; Schulte, M.; Zlotin, S. G. Synthesis of novel tridentate pyrazole-bipyridine ligands for Co-complexes as redox-couples in dye-sensitized solar cells. Tetrahedron 2015, 71 (45), 8551-8556.