Search Thermo Fisher Scientific
Thermo Scientific Chemicals
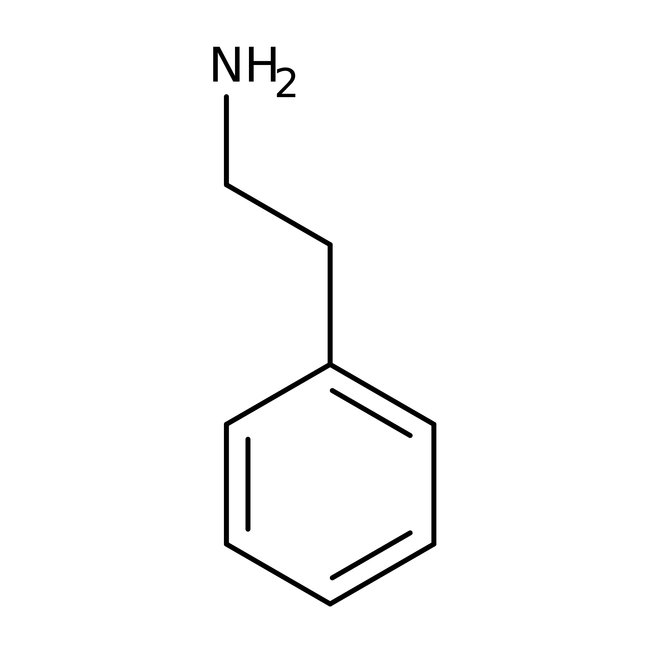
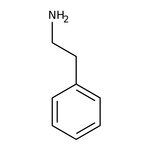
2-Phenylethylamine, 99%
CAS: 64-04-0 | C8H11N | 121.183 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13402.AD | 50 mL |
Catalog number ALFA13402.AD
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 mL
Specifications
Chemical Name or Material2-Phenylethylamine
CAS64-04-0
Health Hazard 1H227-H301-H314-H335
Health Hazard 2GHS H Statement
H301-H314-H318-H227
Toxic if swallowed.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
H301-H314-H318-H227
Toxic if swallowed.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Phenethylamine is used in manufacturing anti-depression agents and anti diabetic drugs. It is also used as the goodds for drug jiangtangling intermediate, also used for other organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenethylamine is used in manufacturing anti-depression agents and anti diabetic drugs. It is also used as the goodds for drug jiangtangling intermediate, also used for other organic synthesis.
Solubility
It is soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Air sensitive. Store under inert gas. Keep away from acids, Acid chlorides, Acid anhydrides, Strong oxidizing agents, Carbon dioxide (CO2).
Phenethylamine is used in manufacturing anti-depression agents and anti diabetic drugs. It is also used as the goodds for drug jiangtangling intermediate, also used for other organic synthesis.
Solubility
It is soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Air sensitive. Store under inert gas. Keep away from acids, Acid chlorides, Acid anhydrides, Strong oxidizing agents, Carbon dioxide (CO2).
RUO – Research Use Only
General References:
- HC Sabelli.; AD Mosnaim. Phenylethylamine hypothesis of affective behavior. American Journal of Psychiatry. 1974, 131,(6), 695-699.
- DA Durden.; SR Philips. Identification and distribution of β-phenylethylamine in the rat. Canadian Journal of Biochemistry. 1973, 51,(7), 995-1002.
- N-Acyl-derivatives are cyclized by a variety of reagents, e.g. P2O5, POCl3, PCl5, etc., to derivatives of isoquinoline, in the Bischler-Napieralski synthesis. For a review, see: Org. React., 6, 74 (1951). Similarly, imines can be cyclized to derivatives of tetrahydroisoquinoline in the Pictet-Spengler synthesis. Reviews: Org. React., 6, 151 (1951); Adv. Het. Chem., 3, 79 (1964); Heterocycles, 3, 223 (1975); 39, 903 (1994).