Search Thermo Fisher Scientific
Thermo Scientific Chemicals
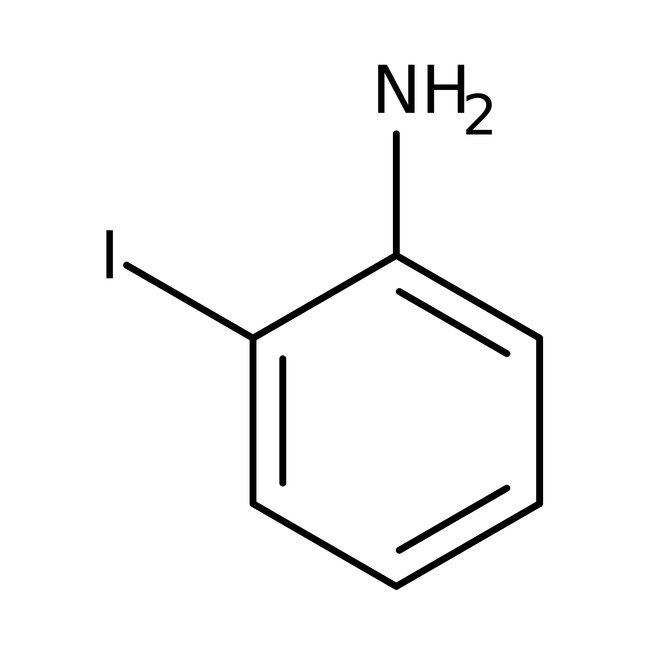
2-Iodoaniline, 98+%
CAS: 615-43-0 | C6H6IN | 219.03 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13059.14 | 25 g |
Catalog number ALFA13059.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material2-Iodoaniline
CAS615-43-0
Health Hazard 1H302+H312+H332-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H311-H332-H315-H319-H335
Toxic if swallowed.
Toxic in contact with skin.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H311-H332-H315-H319-H335
Toxic if swallowed.
Toxic in contact with skin.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
2-Iodoaniline is used as an intermediate in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Iodoaniline is used as an intermediate in organic synthesis.
Solubility
Insoluble in water.
Notes
It is sensitive to light. Incompatible with oxidizing agents.
2-Iodoaniline is used as an intermediate in organic synthesis.
Solubility
Insoluble in water.
Notes
It is sensitive to light. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Gozen Bereket; Evrim Hur; Yucel Sahin. Electrodeposition of polyaniline, poly(2-iodoaniline), and poly(aniline-co-2-iodoaniline) on steel surfaces and corrosion protection of steel. Applied Surface Science. 2005, 252, (5),1233-1244
- Qiuping Ding; Banpeng Cao; Xianjin Liu; Zhenzhen Zongand; Yi-Yuan Peng. Synthesis of 2-aminobenzothiazole via FeCl3-catalyzed tandem reaction of 2-iodoaniline with isothiocyanate in water. Green Chemistry. 2010, (9),1607-1610
- Quinolines may be synthesized by Pd-catalyzed reaction with allyl alcohols: Tetrahedron Lett., 32, 569 (1991).
- Reacts with unsymmetrical acetylenes in the presence of Palladium(II) acetate, 10516, to give indoles substituted at the 2- and/or 3-positions: J. Am. Chem. Soc., 113, 6689 (1991):
- Under similar conditions, ketones with an ɑ-methylene group also give indole derivatives: J. Org. Chem., 62, 2676 (1997).