Search Thermo Fisher Scientific
Thermo Scientific Chemicals
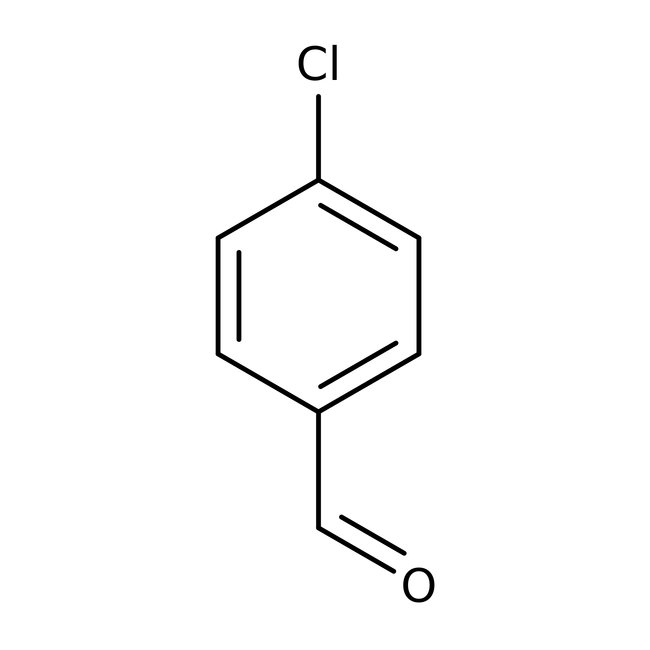
4-Chlorobenzaldehyde, 98%
CAS: 104-88-1 | C7H5ClO | 140.566 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12757.30 | 250 g |
Catalog number ALFA12757.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or Material4-Chlorobenzaldehyde
CAS104-88-1
Health Hazard 1H302-H315-H319-H335-H500
Health Hazard 2GHS H Statement
H302-H315-H319
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
H302-H315-H319
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
4-Chlorobenzaldehyde is used as an intermediate for the manufacture of dyestuffs, optical brighteners, pharmaceuticals, agricultural chemicals and metal finishing products.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Chlorobenzaldehyde is used as an intermediate for the manufacture of dyestuffs, optical brighteners, pharmaceuticals, agricultural chemicals and metal finishing products.
Solubility
Soluble in water (0.94g/L).
Notes
Air Sensitive. Store in cool dry place in tightly closed container. With good ventilation. Store away from air and oxidizing agent.
4-Chlorobenzaldehyde is used as an intermediate for the manufacture of dyestuffs, optical brighteners, pharmaceuticals, agricultural chemicals and metal finishing products.
Solubility
Soluble in water (0.94g/L).
Notes
Air Sensitive. Store in cool dry place in tightly closed container. With good ventilation. Store away from air and oxidizing agent.
RUO – Research Use Only
General References:
- Işul Akmehmet Balcioǧlu; Nicola Getoff. Advanced oxidation of 4-chlorobenzaldehyde in water by UV-light, ozonation and combination of both methods. Chemosphere. 1998, 36(9), 1993-2005.
- Işul Akmehmet Balcioǧlu; Nicola Getoff; Miray Bekbölet. A comparative study for the synergistic effect of ozone on the γ-irradiated and photocatalytic reaction of 4-chlorobenzaldehyde. Journal of Photochemistry and Photobiology A: Chemistry. 2000, 135(2-3), 229-233.
- The nucleophilic (halex) substitution of the chloro substituent by F- can be achieved in good yield by high temperature reaction with KF in tetramethylene sulfone in the presence of Tetraphenyl phosphonium bromide, A15860, as a thermally stable phase-transfer catalyst. This method is general for chloro substituents activated by an ortho- or para-aldehyde group: Chem. Lett., 1355 (1988).