Search Thermo Fisher Scientific
Thermo Scientific Chemicals
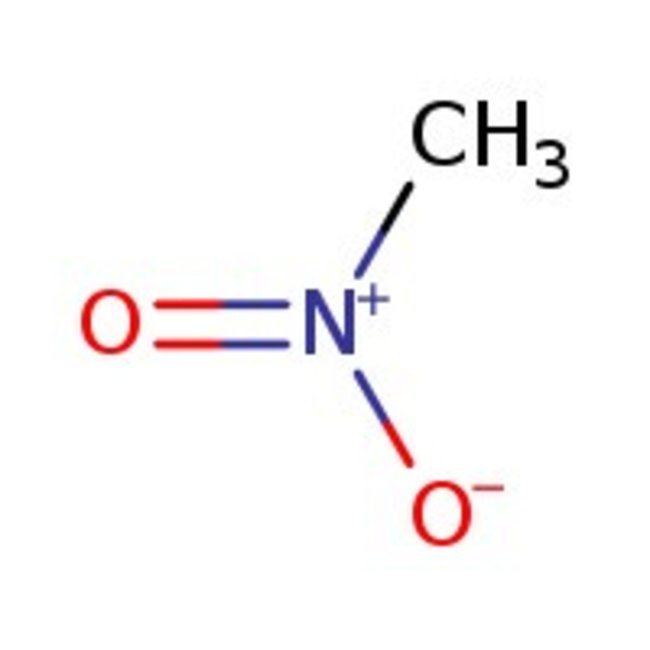
Nitromethane, 98+%
CAS: 75-52-5 | CH3NO2 | 61.04 g/mol
Catalog number ALFA11806.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialNitromethane
CAS75-52-5
Health Hazard 1H226-H302+H332-H350-H361
Health Hazard 3P201-P202-P210-P233-P240-P241-P242-P243-P261-P264b-P270-P271-P281-P301+P312-P303+P361+P353-P304+P340-P308+P313-P330-P370+P378q-P501c
Melting Point°C to 29°C
View more
Nitromethane is used as a stabilizer of halogenated organic solvents, rocket and racing fuel and a chemical intermediate. It is also used as a solvent for cyanoacrylate adhesives, polymers and waxes. It serves as a Michael donor, adding to alfa,beta-unsaturated carbonyl compounds through 1,4-addition in the Michael reaction. It acts as a solvent used for extractions, reaction medium and as a cleaning solvent. Further, it is used in the manufacture of pharmaceuticals, explosives, fibers and coatings.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Nitromethane is used as a stabilizer of halogenated organic solvents, rocket and racing fuel and a chemical intermediate. It is also used as a solvent for cyanoacrylate adhesives, polymers and waxes. It serves as a Michael donor, adding to alfa,beta-unsaturated carbonyl compounds through 1,4-addition in the Michael reaction. It acts as a solvent used for extractions, reaction medium and as a cleaning solvent. Further, it is used in the manufacture of pharmaceuticals, explosives, fibers and coatings.
Solubility
Miscible with ethanol, ethyl ether, acetone, carbon tetrachloride and alkali. Slightly miscible with water.
Notes
Incompatible with amines, strong acids, strong bases, strong oxidizing agents, strong reducing agents and copper
Nitromethane is used as a stabilizer of halogenated organic solvents, rocket and racing fuel and a chemical intermediate. It is also used as a solvent for cyanoacrylate adhesives, polymers and waxes. It serves as a Michael donor, adding to alfa,beta-unsaturated carbonyl compounds through 1,4-addition in the Michael reaction. It acts as a solvent used for extractions, reaction medium and as a cleaning solvent. Further, it is used in the manufacture of pharmaceuticals, explosives, fibers and coatings.
Solubility
Miscible with ethanol, ethyl ether, acetone, carbon tetrachloride and alkali. Slightly miscible with water.
Notes
Incompatible with amines, strong acids, strong bases, strong oxidizing agents, strong reducing agents and copper
RUO – Research Use Only
General References:
- For conditions for the Henry (nitro-aldol) condensation of nitroalkanes with carbonyl compounds, see 1-Nitropropane, A11975 . In the Tiffeneau-Demjanov ring expansion of ketones, the nitro aldol is reduced to the ß-hydroxyamine before diazotization; see, e.g. (cyclohexanone to cycloheptanone): Org. Synth. Coll., 4, 221 (1963); review: Org. React., 11, 157 (1960).
- For a review of the use of nitro compounds as alkyl anion synthons, including methods for removing the nitro group from the resulting carbon skeletons, see: Synthesis, 833 (1988).
- For reaction with TosMIC adducts to give pyrroles, see p-Toluenesulfonyl methyl isocyanide, A14312 .
- For a brief feature on uses of this reagent in Organic synthesis, see: Synlett, 1174 (2007).
- Brequigny, P.; Dayma, G.; Halter, F.; Mounaïm-Rousselle, C.; Dubois, T.; Dagaut, P. Laminar burning velocities of premixed nitromethane/air flames: An experimental and kinetic modeling study. Proc. Combust. Inst. 2015, 35 (1), 703-710.
- Wang, C.; Wu, H. L.; Song, Y. F.; He, X.; Yang, Y. Q.; Tan, D. W. Intense pumping and time-and frequency-resolved CARS for driving and tracking structural deformation and recovery of liquid nitromethane molecules. Chem. Phys. Lett. 2015, 640, 101-105.