Search Thermo Fisher Scientific
Thermo Scientific Chemicals
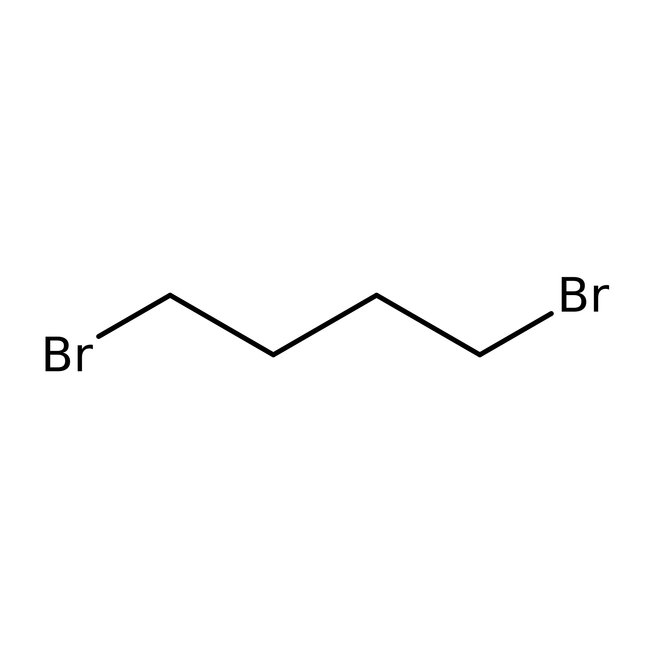
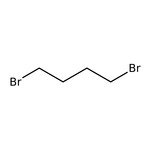
1,4-Dibromobutane, 98+%
CAS: 110-52-1 | C4H8Br2 | 215.916 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11669.36 | 500 g |
Catalog number ALFA11669.36
Price (MYR)
651.00
EA
Quantity:
500 g
Price (MYR)
651.00
EA
Specifications
Chemical Name or Material1,4-Dibromobutane
CAS110-52-1
Health Hazard 1H301-H315-H318-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P305+P351+P338-P310-P312-P330-P332+P313-P362-P501c
View more
1,4-Dibromobutane is used as an intermediate involved in the synthesis of active pharmaceutical ingredient and other organic compounds. It is also used in the investigation of metabolism of two halopropanes such as 1,3-dichloropropane and 2,2-dichloropropane. Further, it acts as a reagent to prepare diazadioxa oxovanadium(IV) macrocyclic complexes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4-Dibromobutane is used as an intermediate involved in the synthesis of active pharmaceutical ingredient and other organic compounds. It is also used in the investigation of metabolism of two halopropanes such as 1,3-dichloropropane and 2,2-dichloropropane. Further, it acts as a reagent to prepare diazadioxa oxovanadium(IV) macrocyclic complexes.
Solubility
Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong bases.
1,4-Dibromobutane is used as an intermediate involved in the synthesis of active pharmaceutical ingredient and other organic compounds. It is also used in the investigation of metabolism of two halopropanes such as 1,3-dichloropropane and 2,2-dichloropropane. Further, it acts as a reagent to prepare diazadioxa oxovanadium(IV) macrocyclic complexes.
Solubility
Immiscible with water.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Chen, J.; Li, N.; Gao, Y.; Sun, F.; He, J.; Li, Y. Dual-responsive polypseudorotaxanes based on block-selected inclusion between polyethylene-block-poly(ethylene glycol) diblock copolymers and 1,4-diethoxypillar[5]arene. Soft matter 2015, 11 (39), 7835-7840.
- Stehouwer, J. S.; Goodman, M. M. Preparation of 1-tosyloxy-4-substituted-2-butenes using Ag(I) salts. Tetrahedron lett. 2015, 56 (30), 4480-4482.