Search Thermo Fisher Scientific
Thermo Scientific Chemicals
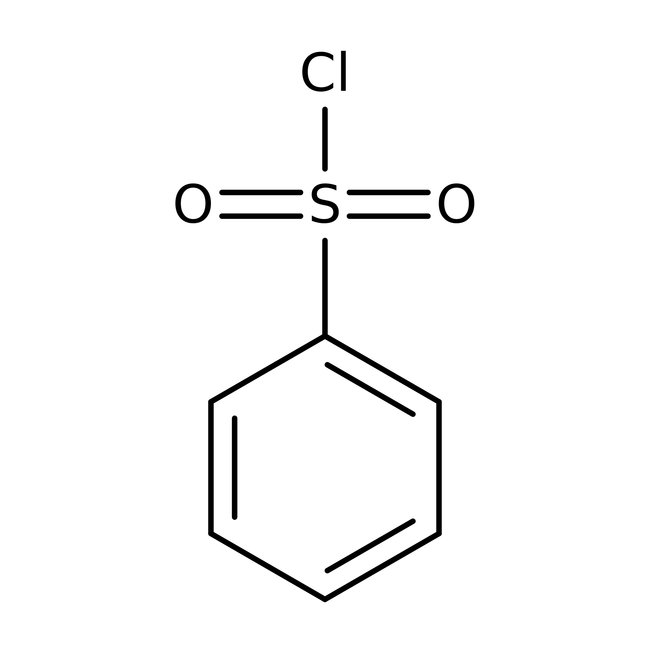
Benzenesulfonyl chloride, 98%
CAS: 98-09-9 | C6H5ClO2S | 176.614 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10849.30 | 250 g |
Catalog number ALFA10849.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialBenzenesulfonyl chloride
CAS98-09-9
Health Hazard 1H302-H314
Health Hazard 2GHS H Statement
H314-H318-H302
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
H314-H318-H302
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
Health Hazard 3P260-P264b-P270-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P501c
View more
Benzenesulfonyl chloride reacts with Grignard reagents to form oxindoles from N-unsubstituted indoles. It is widely used to check the assay of thiamine in different food products. It is involved in the synthesis of alpha-disulfones, sulfonamides and sulfoante esters as precursor. It is a derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry. It is common reagent used in Hinsberg test for detection and distinguishing the type of amines as primary, secondary and tertiary amines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Benzenesulfonyl chloride reacts with Grignard reagents to form oxindoles from N-unsubstituted indoles. It is widely used to check the assay of thiamine in different food products. It is involved in the synthesis of alpha-disulfones, sulfonamides and sulfoante esters as precursor. It is a derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry. It is common reagent used in Hinsberg test for detection and distinguishing the type of amines as primary, secondary and tertiary amines.
Solubility
Insoluble in water. Soluble in ether and alcohol.
Notes
Moisture sensitive. Incompatible with water, strong oxidizing agents, strong bases, methyl formamide and dimethyl sulfoxide.
Benzenesulfonyl chloride reacts with Grignard reagents to form oxindoles from N-unsubstituted indoles. It is widely used to check the assay of thiamine in different food products. It is involved in the synthesis of alpha-disulfones, sulfonamides and sulfoante esters as precursor. It is a derivatization reagent for the determination of various amines in waste water and surface water at the sub-ppb level by gas chromatography-mass spectrometry. It is common reagent used in Hinsberg test for detection and distinguishing the type of amines as primary, secondary and tertiary amines.
Solubility
Insoluble in water. Soluble in ether and alcohol.
Notes
Moisture sensitive. Incompatible with water, strong oxidizing agents, strong bases, methyl formamide and dimethyl sulfoxide.
RUO – Research Use Only
General References:
- Reacts with ß-hydroxy esters in pyridine to give ß-lactones which, at higher temperatures eliminate CO2 in a stereospecific alkene synthesis: J. Am. Chem. Soc., 94, 2000 (1972); J. Org. Chem., 39, 1322, 1650 (1974):
- Alternatively, MgBr2 induces a ring-opening reaction leading to ß-unsaturated acids. See, e.g.: Synth. Commun., 19, 2243 (1989).
- Yuan, K.; Sang, R.; Soule, J. F.; Doucet, H. Desulfitative Pd-catalysed coupling reaction using benzenesulfonyl chlorides and enones as the coupling partners. Catal. Sci. Technol. 2015, 5 (5), 2904-2912.
- Delgertsetseg, B.; Javkhlantugs, N.; Enkhtur, E.; Yokokura, Y.; Ooba, T.; Ueda, K.; Ganzorig, C.; Sakomura, M. Detailed investigation of dependencies of photovoltaic performances of P3HT:PC61BM based solar cells on anodic work function modified by surface treatment of indium-tin-oxide electrode with benzenesulfonyl chloride derivatives. Org. Electron. 2015, 23, 164-170.