Search Thermo Fisher Scientific
Thermo Scientific Chemicals
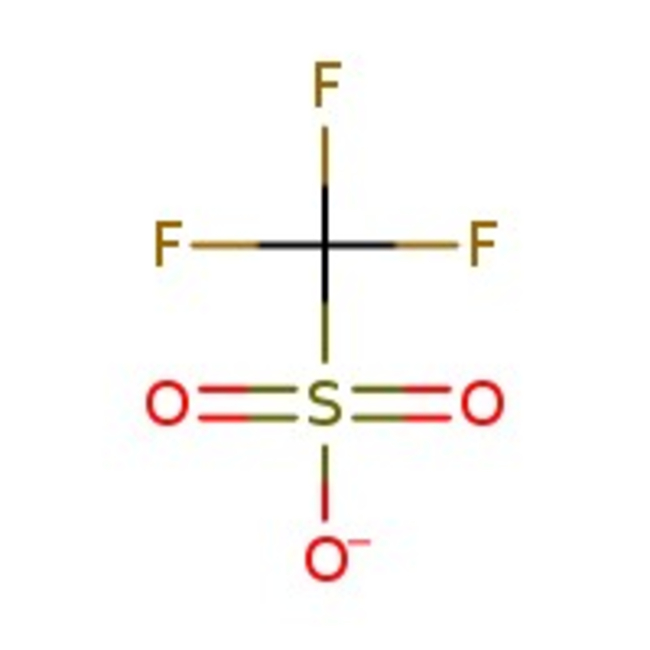
Trifluoromethanesulfonic acid, 98+%
CAS: 1493-13-6 | CHF3O3S | 150.07 g/mol
Catalog number ALFA10173.18
Price (MYR)
832.00
EA
Quantity:
50 g
Price (MYR)
832.00
EA
Specifications
Chemical Name or MaterialTrifluoromethanesulfonic acid
CAS1493-13-6
Health Hazard 1H290-H302-H314-H335
Health Hazard 2GHS H Statement
H314-H318-H302-H335
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
May cause respiratory irritation.
H314-H318-H302-H335
Causes severe skin burns and eye damage.
Causes serious eye damage.
Harmful if swallowed.
May cause respiratory irritation.
Health Hazard 3P234-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P390-P501c
View more
Trifluoromethanesulfonic acid acts as a catalyst for esterification reactions and an acidic titrant in nonaqueous acid-base titration. It is useful in protonations due to the presence of conjugate base triflate is non nucleophilic. It serves as a deglycosylation agent for glycoproteins. In addition, it is a precursor and a catalyst in organic chemistry. It reacts with acyl halides to prepare mixed triflate anhydrides, which are strong acylating agents used in Friedel-Crafts reactions. It acts as a key starting material for the preparation of ethers and olefins by reacting with alcohols as well as to prepare trifluoromethanesulfonic anhydride by dehydration reaction.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Trifluoromethanesulfonic acid acts as a catalyst for esterification reactions and an acidic titrant in nonaqueous acid-base titration. It is useful in protonations due to the presence of conjugate base triflate is non nucleophilic. It serves as a deglycosylation agent for glycoproteins. In addition, it is a precursor and a catalyst in organic chemistry. It reacts with acyl halides to prepare mixed triflate anhydrides, which are strong acylating agents used in Friedel-Crafts reactions. It acts as a key starting material for the preparation of ethers and olefins by reacting with alcohols as well as to prepare trifluoromethanesulfonic anhydride by dehydration reaction.
Solubility
Miscible with water, dimethyl sulfoxide, dimethyl formamide and acetonitrile.
Notes
Hygroscopic. Incompatible with strong oxidizing agents, strong bases, water and metals.
Trifluoromethanesulfonic acid acts as a catalyst for esterification reactions and an acidic titrant in nonaqueous acid-base titration. It is useful in protonations due to the presence of conjugate base triflate is non nucleophilic. It serves as a deglycosylation agent for glycoproteins. In addition, it is a precursor and a catalyst in organic chemistry. It reacts with acyl halides to prepare mixed triflate anhydrides, which are strong acylating agents used in Friedel-Crafts reactions. It acts as a key starting material for the preparation of ethers and olefins by reacting with alcohols as well as to prepare trifluoromethanesulfonic anhydride by dehydration reaction.
Solubility
Miscible with water, dimethyl sulfoxide, dimethyl formamide and acetonitrile.
Notes
Hygroscopic. Incompatible with strong oxidizing agents, strong bases, water and metals.
RUO – Research Use Only
General References:
- One of the strongest available monoprotic acids. For a review of the chemistry of triflic acid and its derivatives, see: Chem. Rev., 77, 69 (1977).
- Simple triflic esters, readily prepared by reaction with alcohols, are powerful alkylating agents and must be handled with extreme care because of their irritating effect on the lungs. For a review, see: Synthesis, 85 (1982).
- For use in direct electrophilic amination of arenes, see Trimethyl silyl azide, L00173. Effects direct oxy-functionalization of aromatics in combination with bis(TMS) peroxide: J. Org. Chem., 54, 1204 (1989); or sodium perborate: Synlett, 39 (1991).
- Adds to both internal and terminal alkynes to give vinyl triflates: J. Am. Chem. Soc., 91, 4600 (1969); Org. Synth. Coll., 9, 472 (1998). Reaction in the presence of a nitrile provides a route to sterically hindered 2,4,6-trisubstituted pyrimidines: Synthesis, 881 (1990):
- Murashige, R.; Ohtsuka, Y.; Sagisawa, K.; Shiraishi, M. Versatile synthesis of 3, 4-dihydroisoquinolin-1 (2H)-one derivatives via intra-molecular Friedel-Crafts reaction with trifluoromethanesulfonic acid. Tetrahedron Lett. 2015, 56 (23), 3410-3412.
- Imai, S.; Kikui, H.; Moriyama, K.; Togo, H. One-pot preparation of 2, 5-disubstituted and 2, 4, 5-trisubstituted oxazoles from aromatic ketones with molecular iodine, oxone, and trifluoromethanesulfonic acid in nitriles. Tetrahedron 2015, 71 (33), 5267-5274.