Search Thermo Fisher Scientific
Thermo Scientific Chemicals
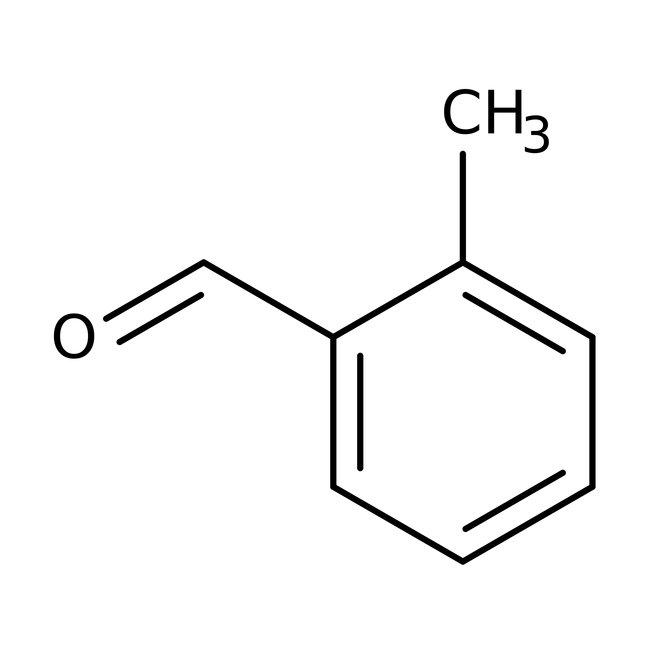
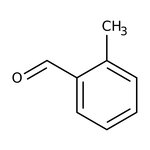
o-Tolualdehyde, 98%, stab. with 0.1% hydroquinone
CAS: 529-20-4 | C8H8O | 120.151 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10172.14 | 25 g |
Catalog number ALFA10172.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Materialo-Tolualdehyde
Name NoteStabilized with 0.1% hydroquinone
CAS529-20-4
Health Hazard 1H227-H302-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H227-H315-H319-H335
Toxic if swallowed.
Combustible liquid.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H227-H315-H319-H335
Toxic if swallowed.
Combustible liquid.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
o-tolualdehyde was used in determination of alkenal-2,4-dinitrophenylhydrazones by HPLC by addition of phosphoric acid. It produces to give a single diastereomer of the tetrahydronaphthalene derivative under UV irradiation.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
o-tolualdehyde was used in determination of alkenal-2,4-dinitrophenylhydrazones by HPLC by addition of phosphoric acid. It produces to give a single diastereomer of the tetrahydronaphthalene derivative under UV irradiation.
Solubility
Soluble in carbon tetrachloride, ethanol, ethyl ether, benzene. Not miscible in water.
Notes
Air Sensitive. Incompatible with air and oxidizing agents. Store under dry inert gas.
o-tolualdehyde was used in determination of alkenal-2,4-dinitrophenylhydrazones by HPLC by addition of phosphoric acid. It produces to give a single diastereomer of the tetrahydronaphthalene derivative under UV irradiation.
Solubility
Soluble in carbon tetrachloride, ethanol, ethyl ether, benzene. Not miscible in water.
Notes
Air Sensitive. Incompatible with air and oxidizing agents. Store under dry inert gas.
RUO – Research Use Only
General References:
- Shigehisa Uchiyama; Erika Matsushima; Shohei Aoyagi; Masanori Ando. Measurement of acid-catalyzed isomerization of unsaturated aldehyde-2,4-dinitrophenylhydrazone derivatives by high-performance liquid chromatography analysis. Analytica Chimica Acta. 2004, 523 157-163.
- Grainne M Clifford; Aurélie Hadj-Aïssa; Robert M Healy; Abdelwahid Mellouki; Amalia Muñoz; Klaus Wirtz; Montserrat Martín Reviejo; Esther Borrás; John C Wenger. The atmospheric photolysis of o-tolualdehyde. Environmental Science & Technology. 2011, 45 (22), 9649-9657.
- Under u.v. irradiation, o-tolualdehyde generates exclusively the (E)-dienol form which may be trapped by cycloaddition with the acrylate (or fumarate) ester of methyl lactate, to give a single diastereomer of the tetrahydronaphthalene derivative: Can. J. Chem., 67, 574 (1989):
- The imine with t-butylamine undergoes lateral lithiation with LDA at the o-methyl group; see, e.g.: J. Org. Chem., 59, 2616 (1994).