Search Thermo Fisher Scientific
Thermo Scientific Chemicals
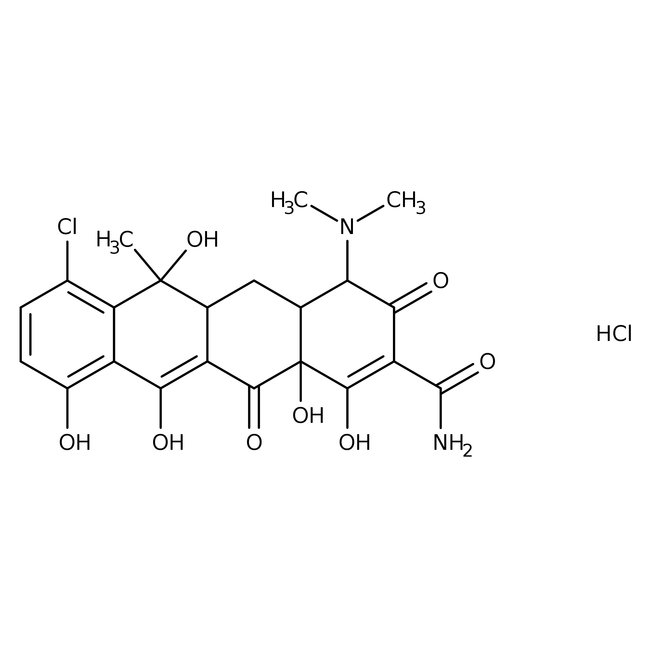
4-Epichlortetracycline hydrochloride, can be used as secondary standard
4-Epichlortetracycline hydrochloride, CAS # 14297-93-9, is a broad-spectrum, bacteriostatic antibiotic of the tetracycline antibiotic family.
Catalog number FSA268231000
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 mg
Packaging:
Glass bottle
Specifications
Chemical Name or Material4-Epichlortetracycline hydrochloride
Name Note97%
CAS101342-45-4
CAS Min %96.0
Health Hazard 1GHS Signal Word: Warning
View more
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General description
• 4-Epichlortetracycline is the hydrochloride salt of 4-Epichlortetracycline, a tetracycline ring structure with a chloride substitution
• 4-Epichlortetracycline hydrochloride (4-Epi-chlortetracycline) is an epimer of chlortetracycline
• 4-Epichlortetracycline suppresses neutrophil activity and dampens the immune response
• Acts as an aminoacyl-tRNA analog to disrupt polypeptide synthesis, reducing bacterial growth Applications
• Can be used for in vitro research on cancer and inflammatory mechanisms
• Intended for use as a selective agent and secondary standard
RUO – Research Use Only
General References:
- Armstrong, A.W.; Hekmatjah, J.; Kircik, L.H. Oral Tetracyclines and Acne: A Systematic Review for Dermatologists. J Drugs Dermatol. 2020, 19(11), s6-s13.
- Liou, J.M.; Chen, C.C.; Chang, C.M.; Fang, Y.J.; Bair, M.J.; Chen, P.Y.; Chang, C.Y.; Hsu, Y.C.; Chen, M.J.; Chen, C.C.; Lee, J.Y.; Yang, T.H., Luo, J.C.; Chen, C.Y.; Hsu, W.F.; Chen, Y.N.; Wu, J.Y.; Lin, J.T.; Lu, T.P.; Chuang, E.Y.; El-Omar, E.M.; Wu, M.S. Taiwan Gastrointestinal Disease and Helicobacter Consortium. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis. 2019,(10), 1109-1120.