Search Thermo Fisher Scientific
Thermo Scientific Chemicals
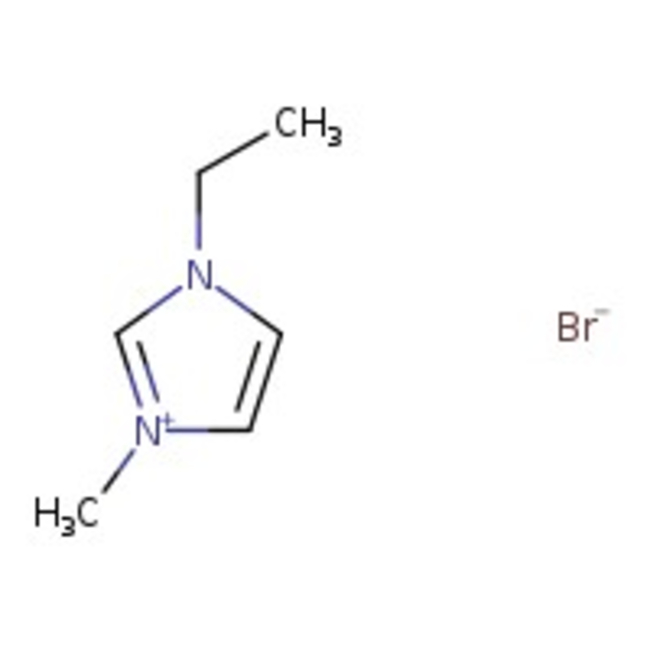
1-Ethyl-3-methylimidazolium bromide, 98+%
CAS: 65039-08-9 | C6H11BrN2 | 191.07 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL19761.18 | 50 g |
Catalog number ALFL19761.18
Price (MYR)
948.00
EA
Quantity:
50 g
Price (MYR)
948.00
EA
Specifications
Chemical Name or Material1-Ethyl-3-methylimidazolium bromide
CAS65039-08-9
Health Hazard 1H315-H319-H335-H500
Health Hazard 2GHS H Statement
H315-H319
Causes skin irritation.
Causes serious eye irritation.
H315-H319
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
An ionic liquid, 1-ethyl-3-methylimidazolium bromide is used as reaction medium for the synthesis and crystallization of a coordination polymer, (EMI)[Cd(BTC)]. 1-ethyl-3-methylimidazolium bromide was used as a solvent in [emim] 2 [Cd 2 (btec)Br 2 ]. Form the diene complexation; carbonyl insertion; cycloaddition; reduction and deoxygenation.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
An ionic liquid, 1-ethyl-3-methylimidazolium bromide is used as reaction medium for the synthesis and crystallization of a coordination polymer, (EMI)[Cd(BTC)]. 1-ethyl-3-methylimidazolium bromide was used as a solvent in [emim] 2 [Cd 2 (btec)Br 2 ]. Form the diene complexation; carbonyl insertion; cycloaddition; reduction and deoxygenation.
Solubility
Soluble in water, dichloromethane, ethanol, acetonitrile. Insoluble in ethyl acetate, ether, and alkane.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents, water, moisture.
An ionic liquid, 1-ethyl-3-methylimidazolium bromide is used as reaction medium for the synthesis and crystallization of a coordination polymer, (EMI)[Cd(BTC)]. 1-ethyl-3-methylimidazolium bromide was used as a solvent in [emim] 2 [Cd 2 (btec)Br 2 ]. Form the diene complexation; carbonyl insertion; cycloaddition; reduction and deoxygenation.
Solubility
Soluble in water, dichloromethane, ethanol, acetonitrile. Insoluble in ethyl acetate, ether, and alkane.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents, water, moisture.
RUO – Research Use Only
General References:
- Mohammed Taghi Zafarani-Moattar,; Shokat Sarmad. Effect of tri-potassium phosphate on volumetric, acoustic, and transport behaviour of aqueous solutions of 1-ethyl-3-methylimidazolium bromide at T = (298.15 to 318.15) K. The Journal of Chemical Thermodynamics. 2010, 42 (10), 1213-1221.
- Steven D. Chambreau,; Jerry A. Boatz,; Ghanshyam L. Vaghjiani,; Christine Koh,; Oleg Kostko.Thermal Decomposition Mechanism of 1-Ethyl-3-methylimidazolium Bromide Ionic Liquid. J. Phys. Chem. A. 2012 , 116 (24), 5867-5876.