Search Thermo Fisher Scientific
Thermo Scientific Chemicals
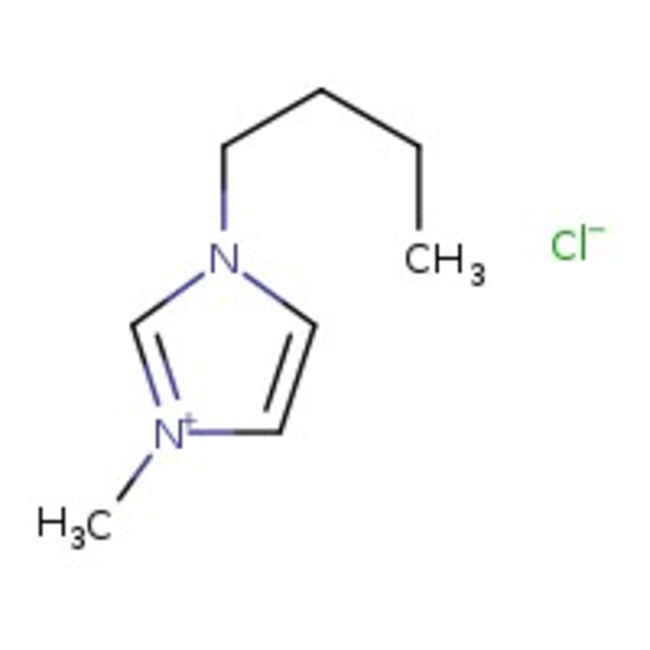
1-n-Butyl-3-methylimidazolium chloride, 96%
CAS: 79917-90-1 | C8H15ClN2 | 174.67 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL19749.18 | 50 g |
Catalog number ALFL19749.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or Material1-n-Butyl-3-methylimidazolium chloride
CAS79917-90-1
Health Hazard 1H301-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
1-n-Butyl-3-methylimidazolium chloride is used as a solvent for organic reactions. It is used in anion exchange reactions. It acts as a catalyst, electrolyte for batteries and metal recovery. It is also used in chromatographic separations. Further, it is used as an ionic liquids for catalysis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-n-Butyl-3-methylimidazolium chloride is used as a solvent for organic reactions. It is used in anion exchange reactions. It acts as a catalyst, electrolyte for batteries and metal recovery. It is also used in chromatographic separations. Further, it is used as an ionic liquids for catalysis.
Solubility
Soluble in acetone, acetonitrile, hot ethyl acetate, isopropyl alcohol, methylene chloride and methanol. Insoluble in water, hexane and toluene,
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong oxidizing agents.
1-n-Butyl-3-methylimidazolium chloride is used as a solvent for organic reactions. It is used in anion exchange reactions. It acts as a catalyst, electrolyte for batteries and metal recovery. It is also used in chromatographic separations. Further, it is used as an ionic liquids for catalysis.
Solubility
Soluble in acetone, acetonitrile, hot ethyl acetate, isopropyl alcohol, methylene chloride and methanol. Insoluble in water, hexane and toluene,
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- In the presence of methanesulfonic acid, alcohols can be converted to alkyl chlorides under mild conditions: Org. Lett., 3, 3727 (2001).
- Men, Y.; Du, X.; Shen, J.; Wang, L.; Liu, Z. Preparation of corn starch-g-polystyrene copolymer in ionic liquid: 1-Ethyl-3-methylimidazolium acetate. Carbohydr. Polym. 2015, 121, 348-354.
- Lu, F.; Wang, L.; Zhang, C.; Cheng, B.; Liu, R.; Huang, Y. Influence of temperature on the solution rheology of cellulose in 1-ethyl-3-methylimidazolium chloride/dimethyl sulfoxide. Cellulose 2015, 22 (5), 3077-3087.