Search Thermo Fisher Scientific
Thermo Scientific Chemicals
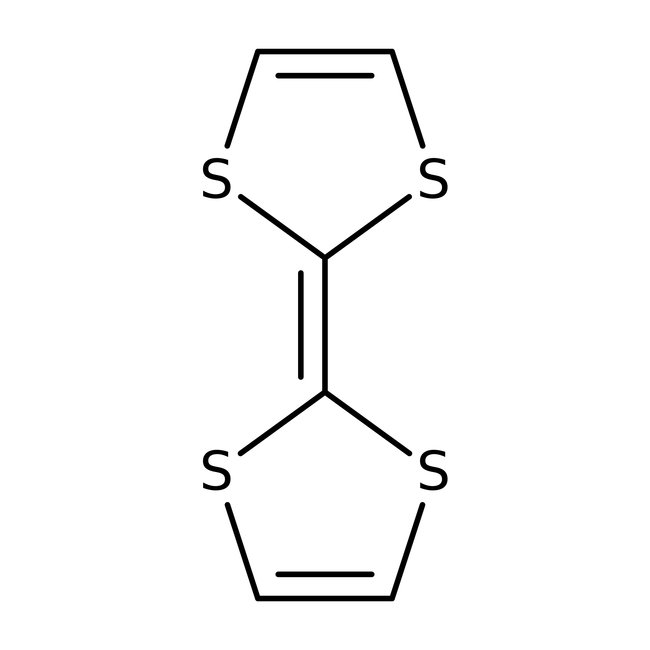
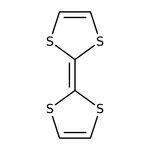
Tetrathiafulvalene, 97%
CAS: 31366-25-3 | C6H4S4 | 204.338 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL19229.03 | 1 g |
Catalog number ALFL19229.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or MaterialTetrathiafulvalene
CAS31366-25-3
Health Hazard 1Warning
Health Hazard 2GHS H Statement
H317
May cause an allergic skin reaction.
H317
May cause an allergic skin reaction.
Health Hazard 3GHS P Statement
P261-P280-P363-P321-P333+P313-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
Wash contaminated clothing before reuse.
Specific treatment (see label).
If skin irritation or rash occurs: Get medical advice/attention.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P261-P280-P363-P321-P333+P313-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
Wash contaminated clothing before reuse.
Specific treatment (see label).
If skin irritation or rash occurs: Get medical advice/attention.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
Tetrathiafulvalene, holds wide application in HPLC, NMR. This heterocyclic compound contributed to the development of molecular electronics. They are used as a organic super conductors.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetrathiafulvalene, holds wide application in HPLC, NMR. This heterocyclic compound contributed to the development of molecular electronics. They are used as a organic super conductors.
Solubility
It is insoluble in water. Soluble in organic solvents.
Notes
Air & Light Sensitive. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents. Stable under recommended storage conditions.
Tetrathiafulvalene, holds wide application in HPLC, NMR. This heterocyclic compound contributed to the development of molecular electronics. They are used as a organic super conductors.
Solubility
It is insoluble in water. Soluble in organic solvents.
Notes
Air & Light Sensitive. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents. Stable under recommended storage conditions.
RUO – Research Use Only
General References:
- JB Torrance.; A Girlando.; JJ Mayerle.; JI Crowley. Anomalous nature of neutral-to-ionic phase transition in tetrathiafulvalene-chloranil. Physical Review Letters. 198147 (24), 1747-1751.
- N Svenstrup.; KM Rasmussen.; TK Hansen.; J Beche. The chemistry of TTFTT. I: New efficient synthesis and reactions of tetrathiafulvalene-2, 3, 6, 7-tetrathiolate (TTFTT): an important building block in TTF-syntheses. Synthesis. 19946 (1), 809-812.