Search Thermo Fisher Scientific
Thermo Scientific Chemicals
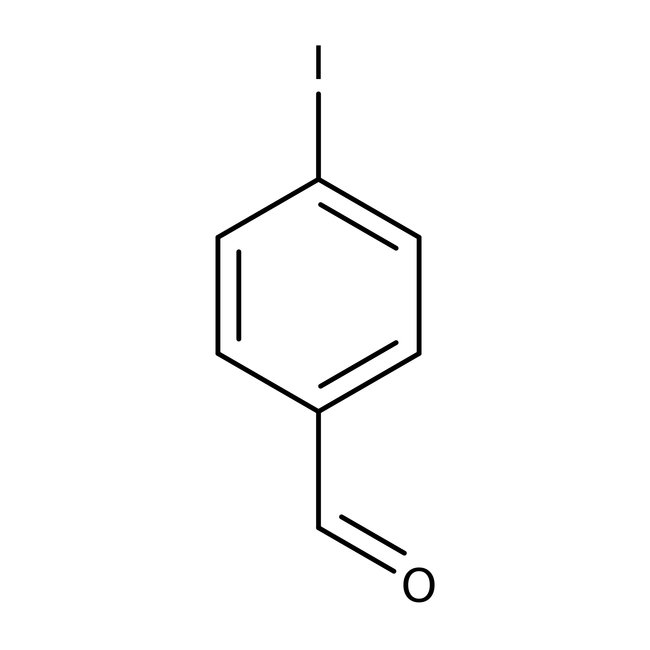
4-Iodobenzaldehyde, 98+%
CAS: 15164-44-0 | C7H5IO | 232.02 g/mol
Catalog number ALFL17448.06
Price (MYR)
2,844.00
EA
Quantity:
5 g
Price (MYR)
2,844.00
EA
Specifications
Chemical Name or Material4-Iodobenzaldehyde
CAS15164-44-0
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
4-Iodobenzaldehyde is used in synthesis of 4-[2-(trimethylsilyl)ethynyl]benzaldehyde, 5,15-dimesityl-10-(3-[2-(trimethylsilyl)ethynyi]phenyl}-20-(4-iodophenyl)porphyrin, and 5,15-dimesityl-10-[3,5-bis{2-[4-(N,N?-difluoroboryl-1,9-dimethyidipyrrin-5-yl)-phenyl]ethynyl}phenyl]-20-(4-iodophenyl)porphyrin.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Iodobenzaldehyde is used in synthesis of 4-[2-(trimethylsilyl)ethynyl]benzaldehyde, 5,15-dimesityl-10-(3-[2-(trimethylsilyl)ethynyi]phenyl}-20-(4-iodophenyl)porphyrin, and 5,15-dimesityl-10-[3,5-bis{2-[4-(N,N′-difluoroboryl-1,9-dimethyidipyrrin-5-yl)-phenyl]ethynyl}phenyl]-20-(4-iodophenyl)porphyrin.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. It is sensitive to air and light. Incompatible with oxidizing agents.
4-Iodobenzaldehyde is used in synthesis of 4-[2-(trimethylsilyl)ethynyl]benzaldehyde, 5,15-dimesityl-10-(3-[2-(trimethylsilyl)ethynyi]phenyl}-20-(4-iodophenyl)porphyrin, and 5,15-dimesityl-10-[3,5-bis{2-[4-(N,N′-difluoroboryl-1,9-dimethyidipyrrin-5-yl)-phenyl]ethynyl}phenyl]-20-(4-iodophenyl)porphyrin.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. It is sensitive to air and light. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Olivier Mongin.; Laurent Porrès.; Laurent Moreaux.; Jerome Mertz.; Mireille Blanchard-Desce. Synthesis and Photophysical Properties of New Conjugated Fluorophores Designed for Two-Photon-Excited Fluorescence. Org. Lett. 2002, 4 (5), 719-722.
- K.J.L. Paciorek.; S.R. Masuda.; J.G. Shih.; J.H. Nakahara. The synthesis of perfluoroalkyl and perfluoroalkylether substituted benzils. Journal of Fluorine Chemistry. 1991, 53 (2), 233-248.