Search Thermo Fisher Scientific
Thermo Scientific Chemicals
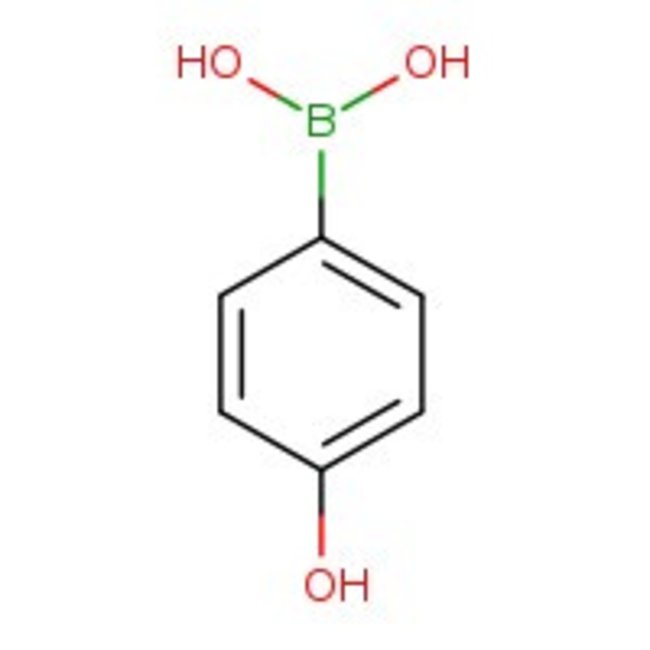
4-Hydroxybenzeneboronic acid, 97%
CAS: 71597-85-8 | C6H7BO3 | 137.93 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL15594.03 | 1 g |
Catalog number ALFL15594.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or Material4-Hydroxybenzeneboronic acid
CAS71597-85-8
Health Hazard 1H302-H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
4-Hydroxybenzeneboronic acid is used for Suzuki-Miyaura coupling and Stille coupling and potential for introducing different alkyl groups, palladium-catalyzed aminocarbonylation and cross-coupling reactions, bio-supported palladium nanoparticles as phosphine-free catalyst for Suzuki reaction in water and Cu2O-catalyzed aerobic oxidative cross-coupling of tetrazoles. It is also used in preparation of rod-like dendronized polymers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Hydroxybenzeneboronic acid is used for Suzuki-Miyaura coupling and Stille coupling and potential for introducing different alkyl groups, palladium-catalyzed aminocarbonylation and cross-coupling reactions, bio-supported palladium nanoparticles as phosphine-free catalyst for Suzuki reaction in water and Cu2O-catalyzed aerobic oxidative cross-coupling of tetrazoles. It is also used in preparation of rod-like dendronized polymers.
Solubility
Soluble in water
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
4-Hydroxybenzeneboronic acid is used for Suzuki-Miyaura coupling and Stille coupling and potential for introducing different alkyl groups, palladium-catalyzed aminocarbonylation and cross-coupling reactions, bio-supported palladium nanoparticles as phosphine-free catalyst for Suzuki reaction in water and Cu2O-catalyzed aerobic oxidative cross-coupling of tetrazoles. It is also used in preparation of rod-like dendronized polymers.
Solubility
Soluble in water
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Mayura Talwelkar.; V.R. Pedireddi. -B(OH)2 versus -OH in supramolecular synthesis: molecular complexes of 4-hydroxyphenylboronic acid with aza-donor compounds. Tetrahedron Letters. 2010, 51 (52), 6901-6905.
- Shunpei Ishikawa.; Kei Manabe. Repetitive Two-step Method for Oligoarene Synthesis through Rapid Cross-coupling of Hydroxyphenylboronic Acids and Anhydrides. Chemistry Letters. 2006, 35 (2), 164-165.