Search Thermo Fisher Scientific
Thermo Scientific Chemicals
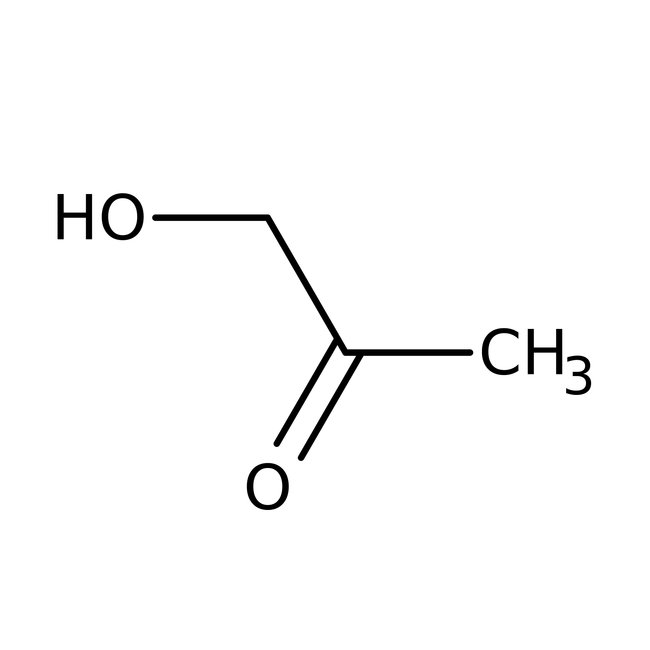
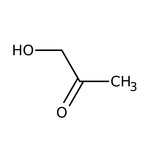
Hydroxyacetone, 95%
CAS: 116-09-6 | C3H6O2 | 74.079 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL15008.22 | 100 g |
Catalog number ALFL15008.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialHydroxyacetone
CAS116-09-6
Health Hazard 1H226
Health Hazard 2GHS H Statement
H226
Flammable liquid and vapor.
H226
Flammable liquid and vapor.
Health Hazard 3P210-P233-P240-P241-P242-P243-P280-P303+P361+P353-P370+P378q-P501c
View more
Hydroxyacetone is used as a reagent in organic chemical reactions. It also serves as a component for Mannich reaction and aldol reactions. It is also used in the syntheses of 2-oxo-propionaldehyde, imidazoles, polyols, acrolein, dyes and skin tanning agents. It yields (R)-1,2-propanediol upon reduction of hydroxyacetone in the presence of a microbial cell catalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hydroxyacetone is used as a reagent in organic chemical reactions. It also serves as a component for Mannich reaction and aldol reactions. It is also used in the syntheses of 2-oxo-propionaldehyde, imidazoles, polyols, acrolein, dyes and skin tanning agents. It yields (R)-1,2-propanediol upon reduction of hydroxyacetone in the presence of a microbial cell catalyst.
Solubility
Miscible with water, alcohol and ether.
Notes
Store in cool place. Air sensitive and hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong acids.
Hydroxyacetone is used as a reagent in organic chemical reactions. It also serves as a component for Mannich reaction and aldol reactions. It is also used in the syntheses of 2-oxo-propionaldehyde, imidazoles, polyols, acrolein, dyes and skin tanning agents. It yields (R)-1,2-propanediol upon reduction of hydroxyacetone in the presence of a microbial cell catalyst.
Solubility
Miscible with water, alcohol and ether.
Notes
Store in cool place. Air sensitive and hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Acetol esters, formed by DCC coupling, have been employed as a means of carboxyl protection, stable to acid and hydrogenolysis, in peptide synthesis. They can be cleaved with TBAF in THF: Tetrahedron Lett., 33, 3193 (1992).
- Albuquerque, E. M.; Borges, L. E.; Fraga, M. A. Lactic acid production from aqueous-phase selective oxidation of hydroxyacetone. J. Mol. Catal. A: Chem. 2015, 400, 64-70.
- St Clair, J. M.; Spencer, K. M.; Beaver, M. R.; Crounse, J. D.; Paulot, F.; Wennberg, P. O. Quantification of hydroxyacetone and glycolaldehyde using chemical ionization mass spectrometry. Atmos. Chem. Phys. 2014, 14 (8), 4251-4262.