Search Thermo Fisher Scientific
Thermo Scientific Chemicals
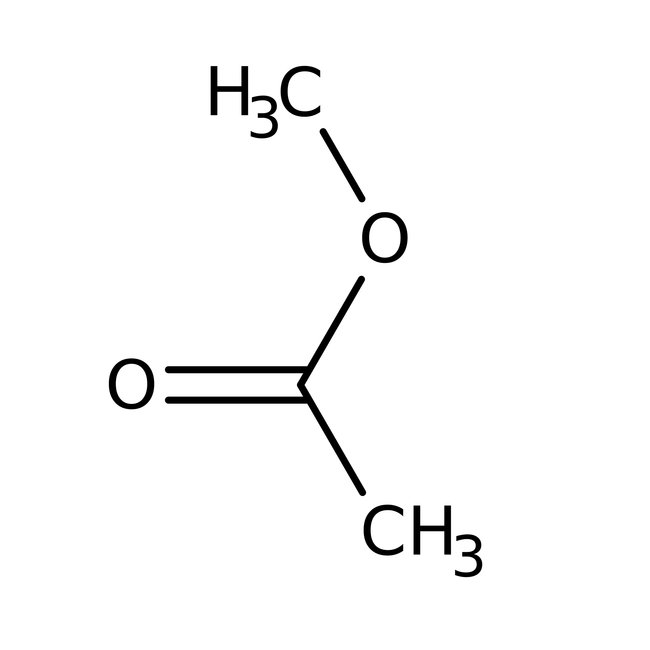
Methyl acetate, 99%
CAS: 79-20-9 | C3H6O2 | 74.079 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL14475.AU | 1000 mL |
Catalog number ALFL14475.AU
Price (MYR)
385.00
EA
Quantity:
1000 mL
Price (MYR)
385.00
EA
Specifications
Chemical Name or MaterialMethyl acetate
CAS79-20-9
Health Hazard 1Danger
Health Hazard 2GHS H Statement
H225-H319-H336
Highly flammable liquid and vapor.
Causes serious eye irritation.
May cause drowsiness or dizziness.
H225-H319-H336
Highly flammable liquid and vapor.
Causes serious eye irritation.
May cause drowsiness or dizziness.
Health Hazard 3GHS P Statement
P210-P261-P303+P361+P353-P305+P351+P338-P405-P501a
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Avoid breathing dust/fume/gas/mist/vapors/spray.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P210-P261-P303+P361+P353-P305+P351+P338-P405-P501a
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Avoid breathing dust/fume/gas/mist/vapors/spray.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
Methyl acetate is used as a solvent in glues, paints and nail polish removers. It is also used in adhesives, sealants and coatings. Further, it finds use as solvent in chemical reactions as well as used for extraction purpose. It is involved in the production of acetic anhydride by carbonylation reaction.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methyl acetate is used as a solvent in glues, paints and nail polish removers. It is also used in adhesives, sealants and coatings. Further, it finds use as solvent in chemical reactions as well as used for extraction purpose. It is involved in the production of acetic anhydride by carbonylation reaction.
Solubility
Miscible with water.
Notes
Moisture sensitive. Incompatible with oxidizing agents.
Methyl acetate is used as a solvent in glues, paints and nail polish removers. It is also used in adhesives, sealants and coatings. Further, it finds use as solvent in chemical reactions as well as used for extraction purpose. It is involved in the production of acetic anhydride by carbonylation reaction.
Solubility
Miscible with water.
Notes
Moisture sensitive. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Yang, X.; Felsmann, D.; Kurimoto, N.; Krüger, J.; Wada, T.; Tan, T.; Carter, E. A.; Kohse-Höinghaus, K.; Ju, Y. Kinetic studies of methyl acetate pyrolysis and oxidation in a flow reactor and a low-pressure flat flame using molecular-beam mass spectrometry. Proc. Combust. Inst. 2015, 35 (1), 491-498.
- Rasmussen, D. B.; Christensen, J. M.; Temel, B.; Studt, F.; Moses, P. G.; Rossmeisl, J.; Riisager, A.; Jensen, A. D. Ketene as a Reaction Intermediate in the Carbonylation of Dimethyl Ether to Methyl Acetate over Mordenite. Angew. Chem. Int. Ed. 2015, 54 (25), 7261-7264.
- Nguyen, H. V. L.; Kleiner, I.; Shipman, S. T.; Mae, Y.; Hirose, K.; Hatanaka, S.; Kobayashi, K. Extension of the measurement, assignment, and fit of the rotational spectrum of the two-top molecule methyl acetate. J. Mol. Spectrosc. 2014, 299, 17-21.
- Niza, N. M.; Tan, K. T.; Lee, K. T.; Ahmad, Z. Biodiesel production by non-catalytic supercritical methyl acetate: thermal stability study. Appl. Energy 2013, 101, 198-202.