Search Thermo Fisher Scientific
Thermo Scientific Chemicals
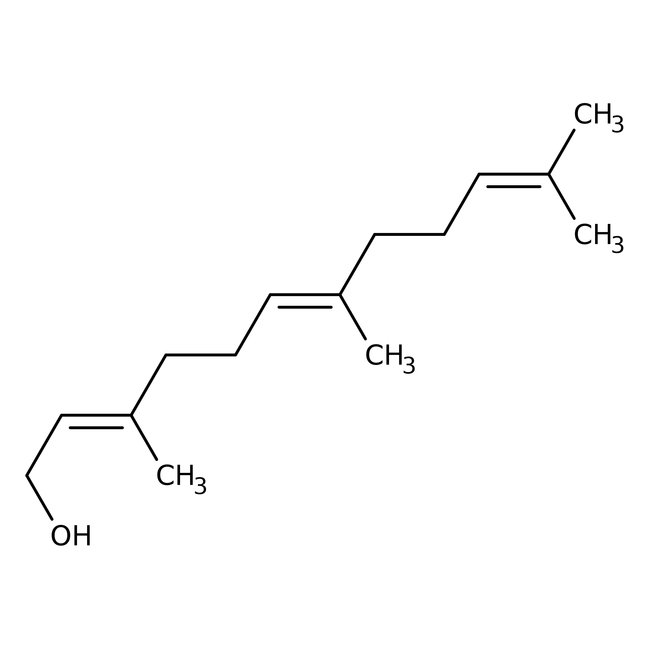
trans,trans-Farnesol, 97%
CAS: 106-28-5 | C15H26O | 222.37 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL14348.06 | 5 g |
Catalog number ALFL14348.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Materialtrans,trans-Farnesol
CAS106-28-5
Health Hazard 1H319
Health Hazard 2GHS H Statement
H319
Causes serious eye irritation.
H319
Causes serious eye irritation.
Health Hazard 3P264b-P280i-P305+P351+P338
View more
trans,trans-Farnesol is an intermediate used in the biological synthesis of cholesterol from mevalonic acid. It is used in the perfume industry. Further, it is used in the preparation of terpenes such as squalenes, cembranoids and forskolin. It is also used in the synthesis of Cecropia juvenile hormone.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
trans,trans-Farnesol is an intermediate used in the biological synthesis of cholesterol from mevalonic acid. It is used in the perfume industry. Further, it is used in the preparation of terpenes such as squalenes, cembranoids and forskolin. It is also used in the synthesis of Cecropia juvenile hormone.
Solubility
Miscible with alcohol. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
trans,trans-Farnesol is an intermediate used in the biological synthesis of cholesterol from mevalonic acid. It is used in the perfume industry. Further, it is used in the preparation of terpenes such as squalenes, cembranoids and forskolin. It is also used in the synthesis of Cecropia juvenile hormone.
Solubility
Miscible with alcohol. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Terpene building block.
- A convenient 2-step alternative to the Gabriel or azide displacement methods for the synthesis of allylic amines has been reported using a Mitsunobu reagent (from Diisopropyl azodicarboxyl ate, L10386) in combination with phthalimide, followed by mild deprotection with methylamine. This technique avoids the allylic rearrangements and other side reactions associated with the older routes: Synthesis, 756 (1995):
- Espinosa, R. R.; Inchingolo, R.; Alencar, S. M.; Rodriguez-Estrada, M. T.; Castro, I. A. Antioxidant activity of phenolic compounds added to a functional emulsion containing μ-3 fatty acids and plant sterol esters. Food Chem. 2015, 182, 95-104.
- Nikolić, B.; Ristić, M.; Bojović, S.; Krivošej, Z.; Matevski, V.; Marin, P. D. Population Variability of Essential Oils of Pinus heldreichii from the Scardo-Pindic Mountains Ošljak and Galičica. Chem. Biodivers. 2015, 12 (2), 295-308.