Search Thermo Fisher Scientific
Thermo Scientific Chemicals
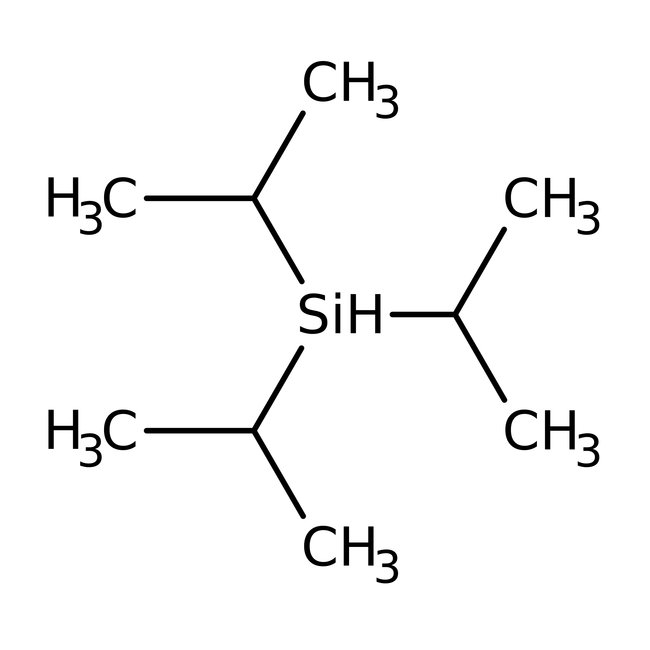
Triisopropylsilane, 98%
CAS: 6485-79-6 | C9H22Si | 158.36 g/mol
Catalog number ALFL09585.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Boiling Point166°C
Chemical Name or MaterialTriisopropylsilane
CAS6485-79-6
Health Hazard 1H226-H315-H319-H335
Health Hazard 2GHS H Statement
H226-H315-H319-H335
Flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H226-H315-H319-H335
Flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
Triisopropylsilane is used as a protecting group in peptide synthesis and is also used as a reducing agent for the selective reduction of anomeric C-phenyl ketals. It is used in the selective silylation of primary hydroxyl groups without affecting the secondary hydroxyl group. It is used in the preparation of anti-1,2-diols catalyzed by a Ni(O) N-heterocyclic carbine complex with high diastereoselectivity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triisopropylsilane is used as a protecting group in peptide synthesis and is also used as a reducing agent for the selective reduction of anomeric C-phenyl ketals. It is used in the selective silylation of primary hydroxyl groups without affecting the secondary hydroxyl group. It is used in the preparation of anti-1,2-diols catalyzed by a Ni(O) N-heterocyclic carbine complex with high diastereoselectivity.
Solubility
Immiscible with water.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids and strong oxidizing agents.
Triisopropylsilane is used as a protecting group in peptide synthesis and is also used as a reducing agent for the selective reduction of anomeric C-phenyl ketals. It is used in the selective silylation of primary hydroxyl groups without affecting the secondary hydroxyl group. It is used in the preparation of anti-1,2-diols catalyzed by a Ni(O) N-heterocyclic carbine complex with high diastereoselectivity.
Solubility
Immiscible with water.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids and strong oxidizing agents.
RUO – Research Use Only
General References:
- Selective silylation of primary OH groups in the presence of secondary has been carried out with this reagent in combination with CsF and imidazole: J. Organomet. Chem., 282, 155 (1985). See Appendix 4.
- Triethylsilane and triisopropylsilane have been studied as cation scavengers in the deprotection of peptides with TFA, and were found to give good results, with triisopropylsilane being particularly effective in trapping trityl cations in the TFA deprotection of cysteine containing peptides. The use of triisopropylsilane minimizes the risk of reduction of the indole nucleus in tryptophan-containing peptides: Tetrahedron Lett., 30, 2739 (1989). See Appendix 6.
- Lee, J. H. Reduction of a 4-pyrrole phenylacyl-containing peptide with trifluoroacetic acid-triisopropylsilane-phenol-H2O during solid-phase peptide synthesis and its protein kinase C alfa inhibitory activity. Bioorg. Med. Chem. Lett. 2005, 15 (9), 2271-2274.
- Aihara, K.; Komiya, C.; Shigenaga, A.; Inokuma, T.; Takahashi, D.; Otaka, A. Liquid-Phase Synthesis of Bridged Peptides Using Olefin Metathesis of a Protected Peptide with a Long Aliphatic Chain Anchor. Org. Lett. 2015, 17 (3), 696-699.