Search Thermo Fisher Scientific
Thermo Scientific Chemicals
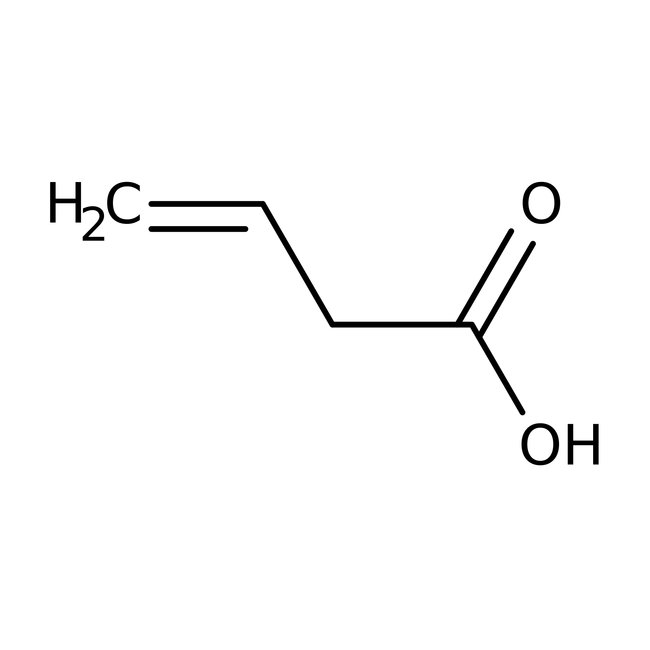
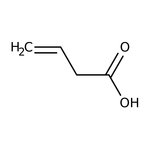
Vinylacetic acid, 96%
CAS: 625-38-7 | C4H6O2 | 86.09 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL07557.14 | 25 g |
Catalog number ALFL07557.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialVinylacetic acid
CAS625-38-7
Health Hazard 1H227-H302-H314-H335
Health Hazard 2GHS H Statement
H314-H318-H227
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
H314-H318-H227
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Vinylacetic acid used in the preparation of strained nitroso Diels-Aldrer adducts which undergo a domino ROC metathesis in the presence of Ru-carbene catalysts and olefins. It is used in the synthesis of polyethylene glycolated boltorn coatings. It was used to study the inhibition of the enzyme-catalyzed conversion of D-tyrosyl- phenylalanyl-glycine to D-tyrosyl-phenylaline amide by non-peptide carboxylic acids.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Vinylacetic acid used in the preparation of strained nitroso Diels-Aldrer adducts which undergo a domino ROC metathesis in the presence of Ru-carbene catalysts and olefins. It is used in the synthesis of polyethylene glycolated boltorn coatings. It was used to study the inhibition of the enzyme-catalyzed conversion of D-tyrosyl- phenylalanyl-glycine to D-tyrosyl-phenylaline amide by non-peptide carboxylic acids.
Solubility
Fully miscible.
Notes
Store at 4°C. Protect from heat. Store away from oxidizing agents. Incompatible with oxidizing agents, heat, Bases, Reducing agents.
Vinylacetic acid used in the preparation of strained nitroso Diels-Aldrer adducts which undergo a domino ROC metathesis in the presence of Ru-carbene catalysts and olefins. It is used in the synthesis of polyethylene glycolated boltorn coatings. It was used to study the inhibition of the enzyme-catalyzed conversion of D-tyrosyl- phenylalanyl-glycine to D-tyrosyl-phenylaline amide by non-peptide carboxylic acids.
Solubility
Fully miscible.
Notes
Store at 4°C. Protect from heat. Store away from oxidizing agents. Incompatible with oxidizing agents, heat, Bases, Reducing agents.
RUO – Research Use Only
General References:
- Marco Laurenti; Pablo Guardia; Rafael Contreras-Cáceres; Jorge Pérez-Juste; Antonio Fernandez-Barbero; Enrique Lopez-Cabarcos; Jorge Rubio-Retama. Synthesis of thermosensitive microgels with a tunable magnetic core.Langmuir.2011, 27 (17), 10484-10491.
- A F Bradbury; J Mistry; B A Roos; D G Smyth. 4-Phenyl-3-butenoic acid, an in vivo inhibitor of peptidylglycine hydroxylase (peptide amidating enzyme).European Journal of Biochemistry.1990, 189 (2), 363-368.
- ß-Unsaturated acids, including vinylacetic acid, undergo bromolactonization with bromine in the presence of bicarbonate giving a mixture of ß- and -lactones: J. Org. Chem., 55, 2487 (1990). Further treatment of the lactone mixture with AgNO3 in acetic acid provides a route to butenolides: Tetrahedron Lett., 34, 1411 (1993).