Search Thermo Fisher Scientific
Thermo Scientific Chemicals
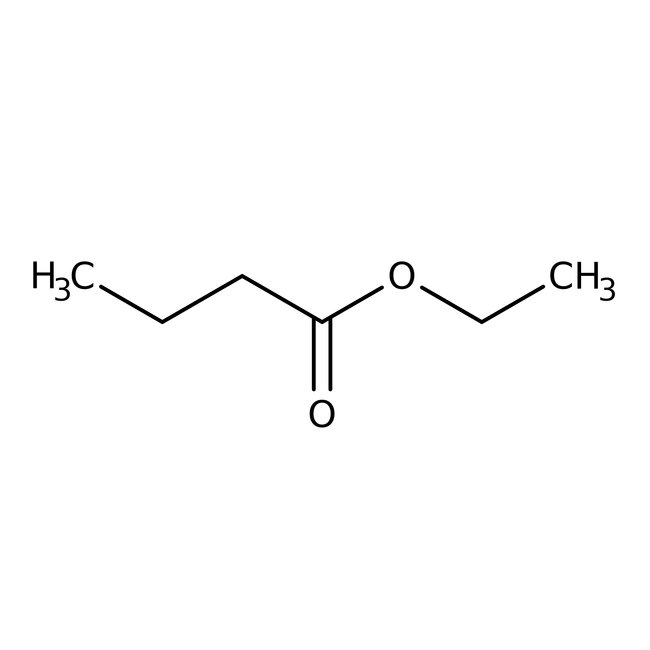
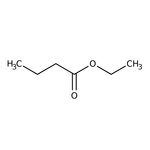
Ethyl butyrate, 99%
CAS: 105-54-4 | C6H12O2 | 116.16 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL06025.AU | 1000 mL |
Catalog number ALFL06025.AU
Price (MYR)
560.00
EA
Quantity:
1000 mL
Price (MYR)
560.00
EA
Specifications
Chemical Name or MaterialEthyl butyrate
CAS105-54-4
Health Hazard 1H226-H312
Health Hazard 2GHS H Statement
H226-H315-H319-H335
Flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H226-H315-H319-H335
Flammable liquid and vapor.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P233-P240-P241-P242-P243-P264b-P280-P303+P361+P353-P333+P313-P363-P374-P380-P501c
View more
Ethyl butyrate is used in flavors and fragrances. It is used as a solvent in perfumery products, and as a plasticizer for cellulose. It is used in the preparation of novel 2-cyanopyrimidines as cathespin K inhibitors. It is also used in the synthesis of pyridobenzimidazole derivatives exhibiting antifungal activity by the inhibition of β-1,6-glucan.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl butyrate is used in flavors and fragrances. It is used as a solvent in perfumery products, and as a plasticizer for cellulose. It is used in the preparation of novel 2-cyanopyrimidines as cathespin K inhibitors. It is also used in the synthesis of pyridobenzimidazole derivatives exhibiting antifungal activity by the inhibition of β-1,6-glucan.
Solubility
Soluble in propylene glycol, paraffin oil, and kerosene.
Notes
Incompatible with oxidizing agents. Store in a cool, dry conditions in well sealed container.
Ethyl butyrate is used in flavors and fragrances. It is used as a solvent in perfumery products, and as a plasticizer for cellulose. It is used in the preparation of novel 2-cyanopyrimidines as cathespin K inhibitors. It is also used in the synthesis of pyridobenzimidazole derivatives exhibiting antifungal activity by the inhibition of β-1,6-glucan.
Solubility
Soluble in propylene glycol, paraffin oil, and kerosene.
Notes
Incompatible with oxidizing agents. Store in a cool, dry conditions in well sealed container.
RUO – Research Use Only
General References:
- Kaitlin S Carlson; Maggie R Dillione; Daniel W Wesson. Odor- and state-dependent olfactory tubercle local field potential dynamics in awake rats. Journal of Neurophysiology.2014, 111, (10), 2109-2123.
- P Sampranpiboon; R Jiraratananon; D Uttapap; X Feng; R.Y.M Huang. Pervaporation separation of ethyl butyrate and isopropanol with polyether block amide (PEBA) membranes. Journal of Membrane Science.2000, 173, (10), 53-59.