Search Thermo Fisher Scientific
Thermo Scientific Chemicals
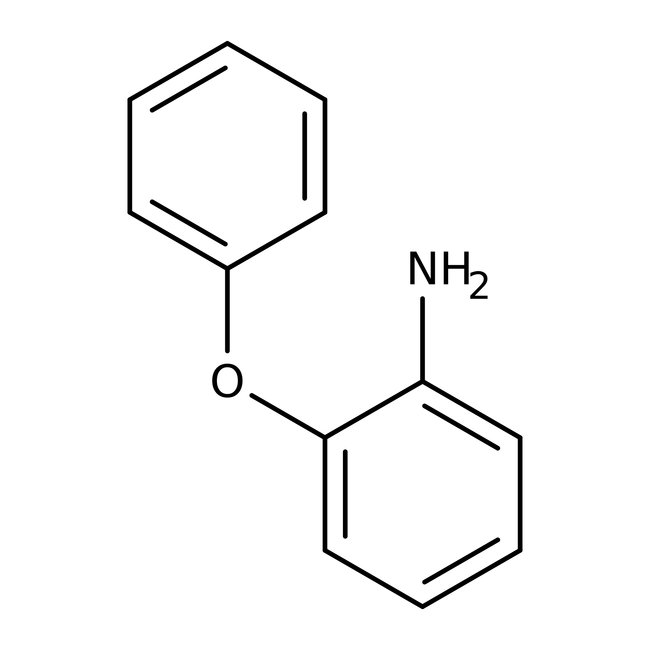
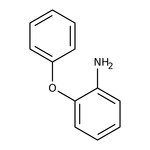
2-Phenoxyaniline, 98%
CAS: 2688-84-8 | C12H11NO | 185.226 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL05558.14 | 25 g |
Catalog number ALFL05558.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material2-Phenoxyaniline
CAS2688-84-8
Health Hazard 1H302+H312+H332-H315-H319-H335
Health Hazard 2GHS H Statement
H301-H311
Toxic if swallowed.
Toxic in contact with skin.
H301-H311
Toxic if swallowed.
Toxic in contact with skin.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
2-Phenoxyaniline serves as an antiinflammatory agent that inhibits preferentially COX-2 over COX-1. It is used in the preparation of sodium primary amide complex, 4-methyl-2-(2-phenoxyphenyl)azo-phenol and 2-acetoaminodiphenyl ether. Further, it is used to form complexes with beta-cyclodextrin.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Phenoxyaniline serves as an antiinflammatory agent that inhibits preferentially COX-2 over COX-1. It is used in the preparation of sodium primary amide complex, 4-methyl-2-(2-phenoxyphenyl)azo-phenol and 2-acetoaminodiphenyl ether. Further, it is used to form complexes with beta-cyclodextrin.
Solubility
Slightly soluble in chloroform and methanol.
Notes
Incompatible with strong oxidizing agents.
2-Phenoxyaniline serves as an antiinflammatory agent that inhibits preferentially COX-2 over COX-1. It is used in the preparation of sodium primary amide complex, 4-methyl-2-(2-phenoxyphenyl)azo-phenol and 2-acetoaminodiphenyl ether. Further, it is used to form complexes with beta-cyclodextrin.
Solubility
Slightly soluble in chloroform and methanol.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Pinheiro, A. C.; Virgili, A. H.; Roisnel, T.; Kirillov, E.; Carpentier, J. F.; Casagrande, O. L. Ni(II) complexes bearing pyrrolide-imine ligands with pendant N-, O- and S-donor groups: synthesis, structural characterization and use in ethylene oligomerization. RSC Adv. 2015, 5 (111), 91524-91531.
- Li, M.; Chen, M.; Chen, C. Ring-opening polymerization of rac-lactide using anilinotropone-based aluminum complexes-sidearm effect on the catalysis. Polymer 2015, 64, 234-239.