Search Thermo Fisher Scientific
Thermo Scientific Chemicals
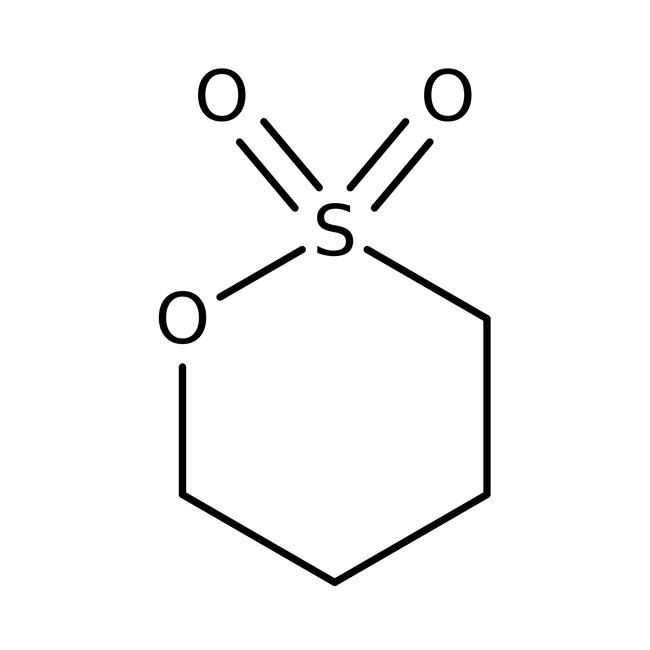
1,4-Butanesultone, 99%
CAS: 1633-83-6 | C4H8O3S | 136.17 g/mol
Catalog number ALFL03992.22
Price (MYR)
849.00
EA
Quantity:
100 g
Price (MYR)
849.00
EA
Specifications
Chemical Name or Material1,4-Butanesultone
CAS1633-83-6
Health Hazard 1H302-H315-H320-H335-H341-H351
Health Hazard 2GHS H Statement
H341-H302
Suspected of causing genetic defects.
Harmful if swallowed.
H341-H302
Suspected of causing genetic defects.
Harmful if swallowed.
Health Hazard 3P201-P202-P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P308+P313-P312-P330-P332+P313-P362-P501c
View more
1,4-Butanesultone is an alkylating agent used in synthetic chemistry. It serves as a wood preservative. It is employed as an intermediate of the organic sulfonation agent. Further, it is used as a raw material for electroplating intermediate. In addition to this, it finds application in active pharmaceutical ingredients, photographic materials, lithium battery and surface active agents.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,4-Butanesultone is an alkylating agent used in synthetic chemistry. It serves as a wood preservative. It is employed as an intermediate of the organic sulfonation agent. Further, it is used as a raw material for electroplating intermediate. In addition to this, it finds application in active pharmaceutical ingredients, photographic materials, lithium battery and surface active agents.
Solubility
Miscible with chloroform and methanol. Slightly miscible with water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents and strong bases.
1,4-Butanesultone is an alkylating agent used in synthetic chemistry. It serves as a wood preservative. It is employed as an intermediate of the organic sulfonation agent. Further, it is used as a raw material for electroplating intermediate. In addition to this, it finds application in active pharmaceutical ingredients, photographic materials, lithium battery and surface active agents.
Solubility
Miscible with chloroform and methanol. Slightly miscible with water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- For a review of the chemistry of sultones, see: Tetrahedron, 43, 1027 (1987).
- Li, G.; Zhao, C.; Li, X.; Qi, D.; Liu, C.; Bu, F.; Na, H. Novel side-chain-type sulfonated diphenyl-based poly(arylene ether sulfone)s with a hydrogen-bonded network as proton exchange membranes. Polym. Chem. 2015, 6 (32), 5911-5920.
- Shen, Y.; Sun, J. K.; Yi, Y. X.; Wang, B.; Xu, F.; Sun, R. C. One-pot synthesis of levulinic acid from cellulose in ionic liquids. Bioresour. Technol. 2015, 192, 812-816.

.png-150.jpg)
