Search Thermo Fisher Scientific
Thermo Scientific Chemicals
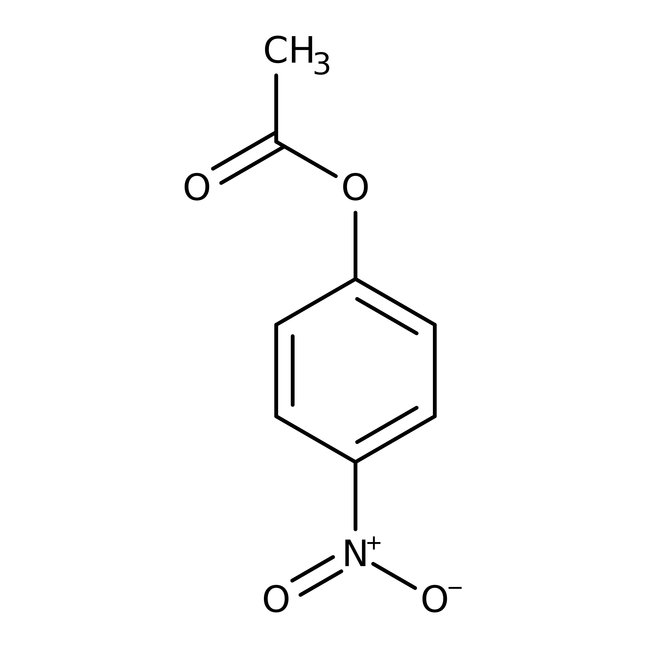
4-Nitrophenyl acetate, 97%
CAS: 830-03-5 | C8H7NO4 | 181.15 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL00314.09 | 10 g |
Catalog number ALFL00314.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Specifications
Chemical Name or Material4-Nitrophenyl acetate
CAS830-03-5
Health Hazard 1H317-H318
Health Hazard 3P261-P272-P280-P302+P352-P305+P351+P338-P310-P333+P313-P363-P501c
Melting Point77°C to 79°C
View more
4-Nitrophenyl acetate is used with iodoacetic acid for reductive cleavage of methionine-containing peptides. It is also used as a substrate that has been used in assays for esterase and lipase activity. Inorganic complexes have been evaluated for their methanolysis or hydrolysis activity using p-nitrophenyl acetate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Nitrophenyl acetate is used with iodoacetic acid for reductive cleavage of methionine-containing peptides. It is also used as a substrate that has been used in assays for esterase and lipase activity. Inorganic complexes have been evaluated for their methanolysis or hydrolysis activity using p-nitrophenyl acetate.
Solubility
Insoluble in water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
4-Nitrophenyl acetate is used with iodoacetic acid for reductive cleavage of methionine-containing peptides. It is also used as a substrate that has been used in assays for esterase and lipase activity. Inorganic complexes have been evaluated for their methanolysis or hydrolysis activity using p-nitrophenyl acetate.
Solubility
Insoluble in water.
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- John C. Hogan.; Richard D. Gandour. Fingerprinting a transition-structure guest by a building-block approach with an incremental series of catalytic hosts. Structural requirements for glyme and .alpha.,. μ. -dimethoxyalkane catalyses in N-methylbutylaminolysis and butylaminolysis of 4-nitrophenyl acetate in chlorobenzene. J. Org. Chem. 1992, 57, (1), 55-61.
- Ik-Hwan Um.; Gwang-Ju Lee.; Hye-Won Yoon.; Dong-Sook Kwon. Solvent effect on rate for anionic nucleophilic substitution reaction of 4-nitrophenyl acetate in aqueous acetonitrile. Tetrahedron Letters. 1992, 33, (15), 2023-2026.
- Reagent for the N-acylation of amines, e.g. basic amino acids: Can. J. Chem., 43, 991 (1965); 46, 1047 (1968).