Search Thermo Fisher Scientific
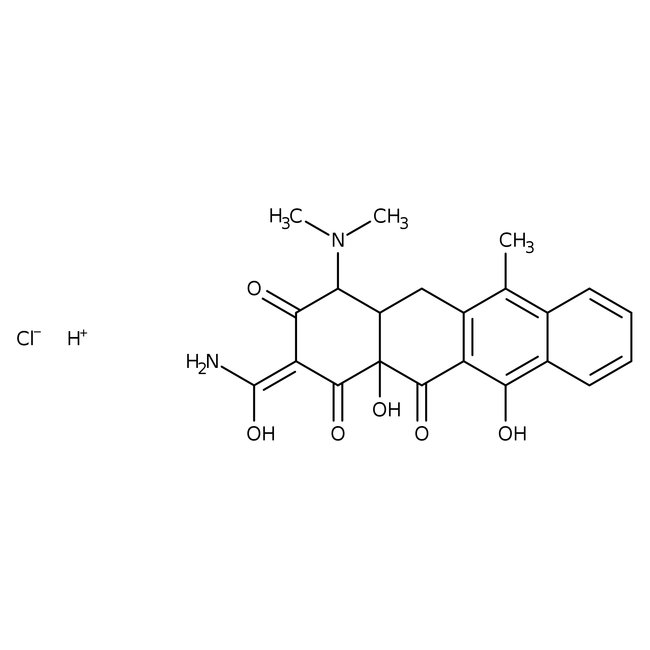
Anhydrotetracycline hydrochloride
| Catalog Number | Quantity |
|---|---|
| ALFJ66688.MA | 10 mg |
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
• Anhydrotetracycline hydrochloride is a degradation product derived from tetracycline hydrochloride. It acts as an effector of tetracycline-dependent gene expression in bacterial expression systems. In this system the transcription of genes is reversibly turned on/off in the presence of several compounds including, anhydrotetracycline hydrochloride.
• This compound poorly binds to the 30S ribosomal subunit compared with its precursor, tetracycline.
Applications
• Anhydrotetracycline hydrochloride is commonly used in all fields of biology for control of transcription or translation processes
• This compound has been used in laboratory experiments and shows lower antibacterial activity against Escherichia coli compared with tetracycline.
General References:
- Baumschlager, A.; Rullan, M.; Khammash, M. Exploiting natural chemical photosensitivity of anhydrotetracycline and tetracycline for dynamic and setpoint chemo-optogenetic control. Nature Communications. 2020, 11, 3834.
- Syed Laik Ali.; Thomas Strittmatter. Separation and quantitative determination of tetracycline, epitetracycline, epianhydrotetracycline and anhydrotetracycline by high-performance liquid chromatography. International Journal of Pharmaceutics. 1978, 1, (3), 185-188.
- Lloyd, P.B.; Carol C. Cornford. A thin-layer chromatographic limit test for the detection of anhydrotetracycline and 4-epi-anhydrotetracycline in tetracycline. Journal of Chromatography A. 1970, 53, (2), 403-405.
- Xie, L.; Wang, C.; Chen, M.; Jin, B.; Zhou, R.; Huang, Y.; Hameed, S.; Ying, Y. Temperature-dependent terahertz vibrational spectra of tetracycline and its degradation products. Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy. 2019, 5, 222, 117179.