Search Thermo Fisher Scientific
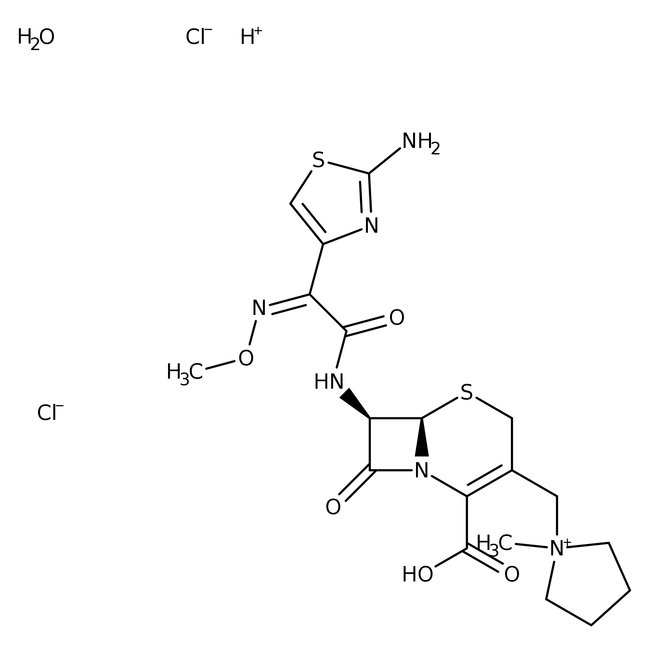
Cefepime hydrochloride monohydrate
| Catalog Number | Quantity |
|---|---|
| ALFJ66237.06 | 5 g |
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
• Cefepime hydrochloride is a fourth-generation cephalosporin antibiotic that works against most antibiotic resistant strains of bacteria
• Cefepime hydrochloride is a penicillin binding protein inhibitor that prevents peptidoglycan cross-linkage and leads to cell wall weakening and lysis
Application
• Cefepime hydrochloride monohydrate can be used for the laboratory study of microbial physiology and antibiotic resistance
• Cefepime has broad-spectrum activity; it is appropriate for research on bacteria responsible for resistant infections and physiological studies
General References:
- Pilmis B, Mizrahi A, Petitjean G, Le Monnier A, El Helali N. Clinical evaluation of subcutaneous administration of cefepime. Med Mal Infect. 2020 May;50(3):308-310. doi: 10.1016/j.medmal.2019.12.006. Epub 2020 Jan 7. PMID: 31924455.
- Rodvold KA, Gotfried MH, Chugh R, Gupta M, Patel A, Chavan R, Yeole R, Friedland HD, Bhatia A. Plasma and Intrapulmonary Concentrations of Cefepime and Zidebactam following Intravenous Administration of WCK 5222 to Healthy Adult Subjects. Antimicrob Agents Chemother. 2018 Jul 27;62(8):e00682-18. doi: 10.1128/AAC.00682-18. PMID: 29784852; PMCID: PMC6105785.